I Don't Think The Word "Quality" Means What You Think It Means
By Ben Locwin, Ph.D.
 The word “quality” is bandied about with reckless abandon across the healthcare, clinical practice, pharmaceutical, and biotech industries. Everyone thinks they understand quality at a deep level, just like everyone thinks they are “better than average” drivers. In fact, no more than 50 percent of the drivers on the road at any given time can be called “better than average.” Just as with driving competency, the reality is that quality expertise, at its best, is normally distributed (meaning half of the industry has a lower-than-average expertise at quality), and at its worst, it is a right-skewed distribution, where only the rightmost tail (representative of maybe 10 percent of the population) has a thorough understanding of the principles, practices, and application of quality concepts. See Figures 1 and 2 below.
The word “quality” is bandied about with reckless abandon across the healthcare, clinical practice, pharmaceutical, and biotech industries. Everyone thinks they understand quality at a deep level, just like everyone thinks they are “better than average” drivers. In fact, no more than 50 percent of the drivers on the road at any given time can be called “better than average.” Just as with driving competency, the reality is that quality expertise, at its best, is normally distributed (meaning half of the industry has a lower-than-average expertise at quality), and at its worst, it is a right-skewed distribution, where only the rightmost tail (representative of maybe 10 percent of the population) has a thorough understanding of the principles, practices, and application of quality concepts. See Figures 1 and 2 below.
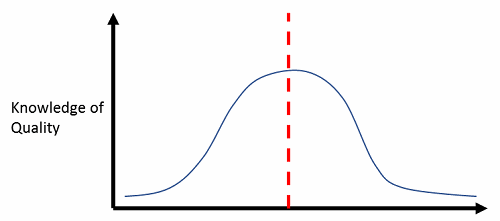
Figure 1: Best-case scenario — half the industry knows less than average about quality principles
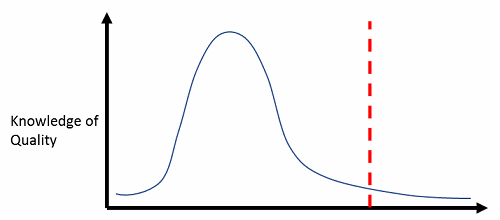
Figure 2: Worst-case scenario — right-skewed distribution, and most staff can function with little understanding
The word “quality” should be very precise in calling to mind certain requirements. Defects, CTQs, rework rates, cost of quality, cost of poor quality, proportion “good” (such as defects per unit and first pass yield), and other specific terminologies give quality its quantitative robustness and repeatability. Once quality is diluted with discussions about inspections and final product quality assessments, it’s not serving its proactive function of ensuring that the processes that are running to (presumably) produce high-quality outputs can be predictably called upon to do what they do without inspections and manual interventions. These are all rework steps and add to the overall cost of quality.
What Is The Cost of Quality?
You may have heard the phrase before, and I’ll explain in more detail here. The cost of quality (CoQ) is comprised of:
- Prevention costs
- Appraisal costs
- Internal failure costs
- External failure costs
Each of these components includes subelements that, in totality, add up to an organization’s cost of “ensuring” some level of quality. Why have “ensuring” in quotes? Because the only way to systematically ensure that an organization’s outputs are of high quality is to design each subordinate process step so that it is repeatable, predictable, and very high quality in and of itself. Then when each process step is chained together in serial (or parallel, depending upon the activities), quantitatively predictable outcomes can be guaranteed to a high degree of certainty. This is the underpinning concept of quality-by-design (QbD). If there are no measurements or quantification of each subordinate step’s actual performance against expected performance, then as each process step completes and leads to the next, the overall outcome is in fact structured around “hope.” Let me be clear: Hope is never a viable long-term quality strategy, but it is invoked at many organizations where proper process characterization seems like a lot of work.
It is not a coincidence that I’ve listed the four components in the order that they are: They are an ordinal list of the most effective ways to apply resources to improve quality.
Establishing specifications for incoming materials and supplies, planning for proactive quality and looking to improve outcomes are prevention costs.
Appraisal costs include measuring and monitoring, such as quality audits, review of intermediate or final products, and supplier (e.g., CRO, CDMO, CMO) ratings.
Internal failure costs are those activities that exist to remediate errors and defects within production process steps. Wasted work and excess inventory (as buffers) exist because of poor characterization of processes. Rework, which refers to doing additional work to correct defects, is an internal failure cost, as is analysis of failure (such as deviation investigation processes). At the tail end of internal failure cost, scrap is defective product that can’t be used (sold) because of significant concerns about its integrity.
And finally, external failure costs refer to costs that exist because defective products or services have reached the customers. This can include injuries, adverse events, complaint resolution, litigation, etc.
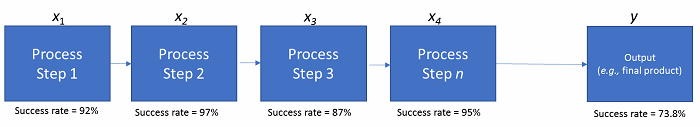
Figure 3: Process steps for rolled throughput yield
In the figure above, what’s called rolled throughput yield (RTY) is demonstrated across a series of fairly high-quality and well-measured process steps. Step 1 has a success rate of 92 percent (~ 1 error per 12 iterations), Step 2 has a success rate of 97 percent, etc. However, with the rules of probability, the overall RTY is the multiplicative product of all of the subordinate steps’ success rates. This leads to an overall outcome success rate of about 74 percent. So, one-quarter of the time, we’ll have an unintended effect or outcome in the final product to explain, justify, or scrap. More steps in a process, nested processes within a sentinel process, and input factors all work together to dramatically increase the level of complexity and decrease the probabilistic level of success for the final product(s).
Pharmaceutical quality isn’t synonymous with quality in potato chip manufacturing. In the food and beverage industry (such as when manufacturing potato chips), defects can be individually discriminated at the end of the manufacturing process and removed singularly (through a fairly sophisticated process, actually). But in pharma, it’s not as inexpensive and easy to strike individual batches from the balance sheet, or vials from the final fill line that don’t adhere to compliance standards. At that point, so much fixed and variable cost, as well as overhead, have been invested in the final product that any quality discard is onerous indeed. Not only that, but if final product defect rates are very high, it’s a trigger for regulatory inspectors to seriously question the quality of the upstream manufacturing processes that led to such a high failure rate. This is not a proactive quality approach using QbD principles.
If you’re not using QbD and don’t have well-characterized performance outcomes for each step in your clinical development or pharma manufacturing processes, you’re operating under a model of “hope.” Plain and simple. Please reach out for help; the future doesn’t need to be that uncertain.
About The Author:
 Ben Locwin, Ph.D., MBA, MS, MBB, is president of Healthcare Science Advisors and is an expert media contact for the American Association for Pharmaceutical Scientists (AAPS) as well as an Advisory Board member for the Healthcare Community of Practice for the Association for Talent Development (ATD). He has worked with top 10 pharma organizations, regulatory agencies (advising on quality and metrics), and in healthcare facilities to improve patient experience and outcomes. You can email him at Ben.locwin@healthcarescienceadvisors.com and follow him on Twitter @BenLocwin.
Ben Locwin, Ph.D., MBA, MS, MBB, is president of Healthcare Science Advisors and is an expert media contact for the American Association for Pharmaceutical Scientists (AAPS) as well as an Advisory Board member for the Healthcare Community of Practice for the Association for Talent Development (ATD). He has worked with top 10 pharma organizations, regulatory agencies (advising on quality and metrics), and in healthcare facilities to improve patient experience and outcomes. You can email him at Ben.locwin@healthcarescienceadvisors.com and follow him on Twitter @BenLocwin.
