Industry Explorers Blaze On: Deborah Dunsire
By Wayne Koberstein, Executive Editor, Life Science Leader
Follow Me On Twitter @WayneKoberstein
Industry Explorers Blaze On: A series of stories of longtime leaders, still active in the industry, sharing their historical perspectives on life sciences industry innovation.

To transform: to start with a scientific hypothesis, use it to create or isolate a potentially therapeutic substance, devise and conduct bench tests, move the compound into preclinical, then clinical trials, and with a great measure of luck, finally gain approval to take the drug to market and make it available to patients. Some people disdain the drug-commercialization process as fraught with fraud and conflict of interest. Others celebrate it as … pure magic. In the latter camp would be Deborah Dunsire, who has lived and worked in a world driven by commercial drug development for three decades.
Dunsire is now president and CEO of tiny XTuit, though her roots are solidly in Big Pharma as a 17-year veteran of Sandoz/Novartis and subsequently head of Millennium pre- and post-Takeda. In her extraordinary career, she has traveled from her original home base in South Africa to Europe and the United States with the eager support of her companies and the people around her. She is aware of the barriers that can confront women executives in the industry, but she has personally sailed right past the most daunting ones, perhaps as an exception that proves the rule. She shows none of the arrogance that you’d think would be required to suggest her experience is something all industry women can have through hard work alone, and her life is full of useful lessons for matching accomplishment to good fortune.
Drawn By Development
Transformation of science to medicine, and the prospect of joining in, served as the compelling force that drew Dunsire into the industry. With a medical degree from the University of the Witwatersrand, she served for a while as a busy family practitioner, then in an emergency room filling time before the planned start of a residency in ophthalmology. Between the two jobs, in 1988, she read a newspaper ad by a pharma company for a job in clinical trials. She had just married and returned from her honeymoon with nine months still to wait for the residency. She thought the clinical research job would be useful for her planned career, and the position came with a company car, which may have tipped the scales. The car alleviated her worry, heightened in her ER experience, about her medical-student husband riding back and forth to the hospital every day on a motorcycle.
Thus, Dunsire joined Sandoz in its South Africa unit headed by Roger Trythall, who soon became her first industry mentor. “Roger hired me pretty much on the spot,” she recalls. “I was starting a new job the next week, and he said, ‘Oh, I’d better make a quick decision about this,’ and before I left from the interview that day, they told me I had the job.”
Dunsire began at Sandoz intending to stay only the nine months until her residency commenced, but the actual experience changed her mind completely.
“I became totally captivated by the impact of scientific knowledge transformed into medicine and how that, in turn, could transform lives,” she says. Her work immersed her in clinical trials for new drugs related to the big Sandoz product, Sandimmune (cyclosporine), for preventing organ rejection in kidney transplantation. There, she says, she observed how the lives of transplant patients changed when stabilized on adequate immunosuppression — something impossible before Sandimmune.
Meanwhile, the CEO offered Dunsire a variety of career paths to contemplate, not in theory but in practice. “He kept saying, ‘We need someone to help train the sales force.’ Or, ‘We need somebody with a good medical background in the clinical research lab to help us work with the commercial plants on a highly specialized product.’ I would say, ‘I’m a physician, what do I know about that?’ And he would just say, ‘I think you can do it, give it a try.’ And so I did.”
While working on the development of Sandostatin (octreotide) for treating the harsh symptoms of some rare tumors, Dunsire relied on advice from many experts in Sandoz around the world to stretch her small budget. A trip to a related conference in the United States offered a unique, long-term opportunity. There she met two other people who became influential in her career: Daniel Vasella, future chairman of Sandoz and Novartis; and David Epstein, who would later head the Novartis Pharmaceuticals Division. Vasella was then the product director, and Epstein, the product manager, for Sandostatin and other drugs.
“It was great to have access to the tools and approaches used in the United States to help expand in South Africa,” says Dunsire. “Daniel and David were very generous with their time and their ideas, and they continued to be mentors in my career. Actually, I worked directly for David Epstein for 11 years when he was head of Novartis Oncology, which was an incredible period of time in Novartis history.”
The meeting portended big changes in Dunsire’s future. Back in South Africa, the more she took on new assignments and higher responsibilities, the more she enjoyed the experience of global drug development. Early on, she had decided to stay with Sandoz rather than return to her planned residency. Several years passed, and once more Trythall pointed the way for her further development. He told her HQ experience would be critical and so, to grow, she moved to Basel, Switzerland, site of the Sandoz headquarters. With his support, she landed an assignment as an international product director, heading the global launch of Sandimmune for autoimmune disease, and she moved to Basel in 1991. Besides taking her into a new therapeutic area, from transplantation to autoimmunity, the job at Sandoz headquarters transported her to a higher level of involvement with the wider world her company and industry covered.
“I recommend to anybody building a career in our industry, if there is an opportunity to work in a different country, take it,” she says. “From a personal growth perspective, it’s an incredible stretch. You have to think about why you do something a certain way and why people in other places do the same thing differently. It challenges your assumptions in so many ways. Global jobs are particularly like that. Not only was I now living in a different country, but I was working across many countries.”
One early lesson: After organizing an international meeting of company experts in Madrid, she found the Spanish custom of late-night meals doomed her plans for a 7 p.m. dinner. “You learn so many things about the cultures, about the people, and about how they live, so it was a very rich time for me in Basel.”
For drug-development strategy, the international differences can be much more challenging and consequential. “At that time, Europe was many countries rather than a single, united region, and we had to deal with how each health authority affected approval and marketing,” she says. “Just bringing together groups of passionate product leaders from all of those countries and navigating through a common strategy together was a true growth experience.”
From Europe, Dunsire’s responsibilities also took her to Japan, where cultural differences can present even steeper challenges in drug development. “For example, there were instances where the regulatory authority would want us to use half the dose that we used in the rest of the world, and we had to manage the best development for patients across countries without creating conflict across the global environment because of it.”
DEBORAH DUNSIRE President & CEO, XTuit
Building Oncology
Dunsire spent several happy years in Basel. But a much larger opportunity, and an even bigger change in location, would soon transform her life again — a move to the United States, along with a move up in professional responsibilities. After taking a VP position at Sandoz U.S. headquarters in New Jersey, she answered a call from Epstein, then leading the Specialty Businesses Division, to begin building an oncology strategy and organization that would eventually have a global reach for Sandoz. “The Sandoz oncology business was initially very small, and then in 1996 all of a sudden we became Novartis!”
Not that the merger of Sandoz and its across-the- Rhine neighbor Ciba-Geigy added much to the oncology franchise. As Dunsire explains, Ciba-Geigy had previously licensed the rights for its biggest cancer drug, Aredia (pamidronate), to Chiron for a period, leaving little of significance in the area. She describes the early days humbly, but with humor: “I always say I was given the opportunity to lead the oncology unit because it was so small and probably didn’t matter much to Novartis!” Fortunately for her fledgling unit, Novartis executed a contract clause that allowed it to bring Aredia back into its product line, which kick-started its oncology business while it developed new compounds in the pipeline. Notably, two of those compounds were Femara (letrozole) and the now legendary Gleevec (imatinib). Throughout Gleevec’s development, Dunsire, Epstein, and the development team worked closely with Alex Matter, the chief scientist for oncology, and Elisabeth Buchdunger, a member of the Sandoz team that helped discover imatinib. The Gleevec project was accelerated by a collaboration with Dr. Brian Druker at Oregon Health & Science University, which began in 1993 with the team at Sandoz led by Dr. Nicholas Lydon. Lydon had left the company before Dunsire’s arrival in Basel.
“Brian Druker and I still are in contact today, I am glad to say,” adds Dunsire, at the same time recalling another enduring connection formed during Gleevec’s development. “Some of the patients from the Phase 1 trial are still alive, and I correspond with one of them, Virginia Garner. Virginia was close to dying, having had CML (chronic myelogenous leukemia) for three years when she went on the Phase 1 trial in 1998. Today, she is still running marathons — half-marathons now — to raise money for the Leukemia & Lymphoma Society. I sponsor the marathons and get her newsletters, and we exchange cards at the holidays. Those cards are some of the most rewarding things in my life, because they remind me I was part of something that made such an incredible difference to so many people whose disease previously would have killed them.”
Until Gleevec, only a few companies had attained any success with new drugs for cancer. For the work-inprogress oncology group at Sandoz/Novartis to score such a victory was frankly astonishing. But Dunsire believes the innovative cultures at Sandoz and Ciba- Geigy, combined in Novartis, explain how the extraordinary feat occurred.
“Sandoz was always a very science-driven company working on medicines that made a difference. It had an eclectic mix of compounds, but they were very innovative. From cylosporin for organ transplantation, all the way down the line to Gleevec, it’s been a very innovative company. Ciba-Geigy had a similar great heritage of innovation. When I hear people criticize Big Pharma about the ability to innovate, I always tell them a lot of the innovative products in the Novartis line came from within. Gleevec, the first targeted agent approved in oncology, went through complete development, from first-in-human to approval by the FDA in only four years and four months, which is unprecedented, because it really works. But it also was because of the company’s commitment to the science and to collaborating with academia and clinical researchers to really fully understand what the drug could do.”
Dunsire helps clarify some of the mythology that has grown up around Gleevec; namely, the widely reported email campaign by patients in the trials that supposedly convinced Vasella to overrule his own scientists and order the product’s development to continue. The emails came after the decision to proceed, she says.
“David Epstein and I went to the New York offices to talk to Daniel about continuing the development after analyzing the market and finding it would be small. How wrong were we? But we said the data is so good, we can’t walk away. So we agreed in that meeting, even though the forecast for the market was tiny, that there was nothing else for us to do but keep going. The email campaign actually came further down the line. We didn’t have a commercially scaled process by the time we knew the drug was spectacularly effective, so we didn’t have enough supply for all the patients who needed to be on the drug. We had to delay the Phase 2 trial to make sure the Phase 1 patients could continue to get the drug, and that caused a lot of email traffic from patients, which was empathetically received, but it wasn’t the difference between the drug proceeding or not; that decision already had been made.”
For all of its value, Gleevec may have obscured a broader accomplishment on Dunsire’s watch — building the oncology organization practically, as one biography states, “from the ground up.”
DEBORAH DUNSIRE President & CEO, XTuit
How do you build organizations from the ground up — especially after a major merger? Start by keeping or bringing in people with the right skills and motivation, she says, then adds a realistic caveat:
“If you can bring on a team of people who are passionate about transforming outcomes for patients, it is easier to unite around that goal and create the new culture. With that focus, the conventional wisdom on big-company mergers — as in 30 percent of the staff will turn over before the different company cultures really start to meld — can be mitigated.”
With the oncology group, Dunsire’s challenge was even greater because she inherited no definite plan. That was the “from the ground up” part. Instead, she says, “It was a constant surprise for me. Novartis Oncology is now over a $10 billion business, but when it started out, there was no specific corporate strategic plan to build an oncology business or to invest in oncology as a company pillar. It was driven by doing the right thing for the patients and having innovative products in the pipeline. We came together to deliver transformative medicines, and gradually the organization emerged. I was privileged to be its leader.”
Millennial Leap
Dunsire remained at Novartis oncology for many years and led the development of numerous other drugs in the cancer area. Not all of them treated cancer itself; a few addressed ancillary conditions such as tumorcaused fractures. As she describes, it was not a topdown, command-and-control portfolio, but a variety that genuinely reflected the relatively opportunistic style of the company and the oncology organization built under Dunsire’s leadership.
Nothing, as they say, lasts forever, and the best things in life never last long enough. When Dunsire answered an outside opportunity after 17 years at her first and only company, one could argue it was a good time to move on — because she was sorely needed in a much younger sector of the industry. In 2005, when she took the CEO position at Millennium, that company wasn’t performing as expected. Most people around the industry at the time will remember that Millennium’s single major product, Velcade (bortezomib), had fallen far short of projections and its sales had plateaued in its only approved indication, relapsed/refractory myeloma. But the company had great people and a promising pipeline, and Velcade still had a much larger potential.
Not that the decision to leave Novartis came easily. Dunsire knew she wanted to extend the reach of her responsibility from the commercial area to include the span of R&D. She was already covering much of that ground in her position at Novartis and envisioned herself as the chief executive of an entity that contained the entire span of functions from science to market. In the traditional career path at Novartis, her next step would likely have been running a regional division, such as South America. Yet, even though that role would have increased her commercial role tremendously, it would also have encompassed the company’s entire product line, not just the innovative side. “I’ve always wanted to work on the leading edge of therapeutics for diseases that are truly unmet by the available medicines,” she says.
As she was contemplating her next move, she received a call from a recruiter who offered her a job that fit all of her criteria. “Millennium had all of those elements. It was focused in oncology and inflammatory diseases, and it had a reputation for phenomenal science, drug discovery programs, a pipeline in development, and a transformative oncology product already on the market.”
![]()
“I honestly can’t identify an obstacle I’ve faced that was driven by me being a woman, although I’ve certainly faced obstacles.”
DEBORAH DUNSIRE
President & CEO, XTuit
But that major oncology product needed help, and the company needed more commercial clout. Founding CEO Mark Levin, who was the first person Dunsire met at Millennium, moved quickly to introduce her to his team and place her in his job. “Mark was an iconic leader, and the people within the company loved him. He made the transition easy for me because he told them, ‘We need a new leader for this time in the company’s life. We know we’ve got to be more successful commercially, and I believe this is the person who can help us do that without losing focus on driving our science forward.’ Watching Mark, I learned so much about vision, how people are inspired, and how extraordinary things are accomplished when people have a vision. I’m eternally grateful to him.”
After the meeting with Levin and the company staff, Dunsire met with the members of the board. First was Dr. Eric Lander at the Whitehead Institute in Cambridge, MA, a persistent driver of academic-to-business startups and outspoken advocate of new technologies such as CRISPR. “Eric offered me tea — real English tea. Of course, he had been a Rhodes Scholar at Oxford, where he studied mathematics. The entire Millennium board was made up of stellar individuals. I was so privileged to work with them in my first CEO role.”
In her first days at the company, she focused on the commercial organization. “Millennium was a company deeply rooted in the science. They had the idea that science is hard, and once the science is good, everything else is easy. But they had come to realize that wasn’t necessarily the case. I guess my job was to tell them it’s all hard. Science is indeed hard but commercialization requires the same hard effort and the same degree of outstanding talent. At the same time, even with my commercial background, they could trust me not to obliterate the products of the future, but to nurture the development pipeline.”
When Dunsire came on board, Millennium was divesting all of its core cardiovascular assets, including Integrilin (eptifibatide), to its partner Schering Plough. She says cocommercializing the products would have made them even more of a distraction than in-house management, blurring the company’s focus on oncology and ability to recruit the right team in that area. “I sound like a broken record, but with Velcade, we ultimately attracted a number of people extremely enthusiastic about leading the commercial team. I was able to hire Dr. Christophe Bianchi from Sanofi, and he’s an extraordinary commercial leader. With Velcade, the solution was getting the right team, simplifying the way it was promoted, expanding the sales force — doing things that I had done for years.” And as others have noted, subsequent approvals moved Velcade from third-line to first-line use. “Velcade is one of those life-transforming products for myeloma patients. It grew up into an incredibly successful brand, now close to $4 billion worldwide.”
Dunsire also fulfilled her commitment to moving new drugs through the pipeline and into the market. One was vedolizumab (Entyvio), the antibody of integrin α4β7 (LPAM-1, lymphocyte Peyer's patch adhesion molecule 1), for ulcerative colitis. “Vedolizumab is an extraordinary drug, and it might never have happened because it was partnered with Genentech and neither one could figure out how to make a stable cell line. Eventually, Genentech gave the product back, along with some very good clinical data the two companies had on the drug. I looked at that data and said, ‘This drug works. We’ve got to find a way to do this.’ But I said to the team, ‘We can’t spend money on it forever. You’ve got six months. If you can figure out the cell line, then we’ll go forward.’ The protein science team at Millennium did figure it out, and after many ups and downs, the product came to market.”
An oral proteasome inhibitor to treat relapsed or refractory multiple myeloma was the next challenge. Dunsire and the R&D team debated at length about what form the product should take and the researchers explored the alternatives. “Eventually, we found the right answer and our R&D brought forward Ninlaro [ixazomib], the world’s first oral proteasome inhibitor.”
Financing all of the product development led Dunsire and the company to realize it had to be more selective in R&D, which required defining the right focus in the R&D portfolio. Beyond that, she says the overarching challenge was rebuilding the confidence of the company. “When I took over there was a 22 percent employee turnover rate, and by the time of the Takeda acquisition, it had come down to well below the industry average and for years following the acquisition by Takeda in 2008, we had about 3 percent turnover.”
Takeda Takeover
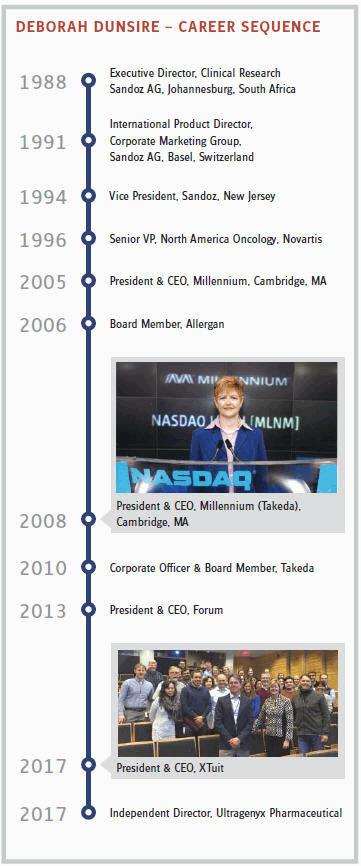 A mention of the Takeda acquisition, ephemerally creating “Millennium: The Takeda Oncology Company,” marks the next big stage in Dunsire’s career. All of my interviews with the principals seem to support this interpretation of the merger: The acquired had more influence on the acquirer than the other way around. It was primarily culture, not only commerce, that drove the deal. To no surprise, then, the acquisition of Millennium by Japan’s oldest pharma company began with a personal connection that led to a meeting of minds.
A mention of the Takeda acquisition, ephemerally creating “Millennium: The Takeda Oncology Company,” marks the next big stage in Dunsire’s career. All of my interviews with the principals seem to support this interpretation of the merger: The acquired had more influence on the acquirer than the other way around. It was primarily culture, not only commerce, that drove the deal. To no surprise, then, the acquisition of Millennium by Japan’s oldest pharma company began with a personal connection that led to a meeting of minds.
Dunsire and Anna Protopapas, then head of business development, traveled to Japan to visit companies, looking for opportunities for potential in-licensing of products, partnering, research funding, and so on. One of the companies was Takeda, where they met then-president and subsequently CEO and chairman, Yasu (Yasuchika) Hasegawa.
“Yasu was truly a visionary leader of the company,” she says. “If there’s an inner transformation at Takeda, it was because he personally drove it.” Hasegawa had lived in the United States for 10 years and Germany for three years, and had a global vision for Takeda. At first, Dunsire and Protopapas believed Takeda’s only interest was in Millennium’s science. They arranged a number of discussions between the two companies’ scientific teams but never found the right formula for a partnership that worked ideally for both of them.
“Then Yasu Hasegawa asked if he could meet us during JP Morgan at a nearby restaurant. He said, ‘Look, we’ve really thought about this, and for us to become a leader in oncology, we need a major catalyst, and we believe that bringing Millennium into Takeda could be that catalyst. We would want Millennium to be there for our oncology line.’”
Dunsire told him the timing might be difficult because Millennium was preparing for a big launch of the firstline indication for Velcade. “I said, ‘We have to be successful, I can’t distract people. I understand that Japanese companies can be very diligent, which can take a long time. I’m not prepared to put the company through a long process. How quickly can you do this?’ And he said to me, ‘Six weeks.’ And lo and behold, it was six weeks.”
Hasegawa proclaimed he was not buying Velcade; he was bringing Millennium in to be a transformation agent for Takeda. Dunsire said he told her, “‘We need to become more global in our thinking. We need to transform, and not only can Millennium build our oncology, but also catalyze our transformation.’ We were afforded the opportunity to lead oncology and to be that transforming agent within Takeda. I credit that vision to Yasu Hasegawa.” (See our June 2016 feature article, “Takeda's New Plans For Worldwide Growth.”)
In the five years following the acquisition, Dunsire remained at the helm of Millennium, now Takeda Oncology, leading it through an impressive period of growth. It maintained a healthy pipeline that produced a run of good products and augmented it with business development. She mentions one deal in particular, a partnership with Seattle Genetics on brentuximab vedotin (Adcetris), a successful chemotherapy for treating Hodgkins and anaplastic large cell lymphomas, with Takeda as the commercial partner. The deal connected her to Seattle Genetics’ CEO, Clay Siegall, who later supported her entry to the board of Ultragenyx, a developer of therapeutics for ultra-rare diseases. Dunsire also was elevated to a board position in Takeda — a stunning first for a woman in the company.
Ultragenyx may have awakened her startup spirit, or perhaps it was all of those long flights to and from Japan, but no question Dunsire was eventually drawn away from the corporate world of Takeda as she had been from Novartis years earlier. She left the company in good reputation and took the CEO position at little Forum Pharmaceuticals, which was fighting an ultimately losing battle to develop a drug for schizophrenia and dementia.
Forum was a private, precommercial company going into Phase 3 clinical trials when she joined it in 2013. Forum’s investors were all at a single firm, Fidelity, and they wanted to build a new model for biopharma companies by showing they could insulate Forum from the need for further investment or partnering as it commercialized the pipeline. “It was a mouth-watering challenge to take on Alzheimer’s and then build the company around it, with the opportunity to bring the drugs through development and into the commercial realm in a new way,” says Dunsire. “I had to really make the company transform from a scrappy early-stage organization and rapidly expand the operation for running four global Phase 3 clinical trials. Yet I had to be careful not to lose the essence of the company. You have to be very diligent to ensure that you build the culture you want and you retain the essence of what makes the company great and what drives people to come to the company and stay there.”
After a Phase 3 trial produced poor results, however, the company folded. Dunsire has opined elsewhere on why that happened, including a possible flaw in how patients were assessed for progress. Yet, as a couple of years of failed Alzheimer’s trials in multiple companies attest, no one has identified an unerring or even roughly indicative set of biomarkers for the disease.
Following Forum, Dunsire got right back on the horse in her current job as CEO of XTuit which has taken on therapy-blocking fibrosis in cancer. “XTuit is the earliest company I’ve run, and it’s like being in a small boat, very close to the ocean so you can feel the waves,” she says. “If you’re on an ocean liner, you look down and the waves are somewhat distant. Here I have the fun of creating the team, leaning into the science, and taking it forward. There is a lot of excitement, and a lot of roller coaster feelings as well.”
She came to XTuit with a lead from a longtime colleague at Millennium, Dr. Alan Crane, the founding investor of the company’s drug platform for clearing the tumor microenvironment of fibrotic tissues that could keep other therapeutic agents from reaching the cancer. “This company is focused on normalizing the aggressive microenvironment to reverse fibrosis and make cancers treatable, and that’s a big mission,” Dunsire says. The founding biology comes from the world-leading labs of Dr. Rakesh Jain at Harvard/MGH and Dr. Ron Evans at the Salk Institute. Dr. Robert Langer at Harvard, who cast the model for enterprises spun from academic science, is also a founder.
Big missions, big challenges, and of course, big transformations still drive this industry explorer on to blaze new trails. Like many other industry players who have been around to see a good slice of history, she is obviously not done yet — not by a long shot.
One Woman’s Industry Journey
Even though Deborah Dunsire has done extremely well as (not for) a woman in the traditional man’s world of Big Pharma, she still sees institutional and cultural barriers to the advancement of women in the industry. In the following, she describes her experience and observations of the issue in her 30-year career.
I honestly can’t identify an obstacle I’ve faced that was driven by me being a woman, although I’ve certainly faced obstacles. But can I see it in the industry and do I see women who have been affected? Yes, I do. One of the things that I’ve faced, perhaps without realizing it, is the difference in how women are perceived in a conflict situation compared to men. If there’s conflict, a man is seen as strong, but a woman may be seen as strident. Have I seen improvement? Oh, yes, I certainly have. There are more women in CEO roles and more first-time female CEOs now than there were 10 years ago. When I came to Cambridge, there were maybe two other female CEOs in the biotech space. The landscape has shifted. Of course, there’s been an incredible, unprecedented rate of companies starting up in the last 10 years, particularly in the last five years, but we also have more people who are open to seeing qualified women as able to lead and raise money for an organization. There is the odd pocket of male-chauvinist behavior — but thankfully few and far between.

