Singapore's Evolution To A Top Phase 1 Trial Destination
By Christophe Tournerie and Yew Lay Hwa
Singapore can be considered as the R&D spearhead in Asia with cutting-edge technology, top clinicians, a solid and advanced infrastructure which rivals the West, as well as drive, commitment, and investment by its government to become one of the preferred clinical research destinations worldwide. The country plays a substantial role in conducting Phase 1 studies in the Asia-Pacific region. Many pharmaceutical and biotech companies are now choosing to bring their innovative early-phase trials to Singapore over the more conventional options of the United States or Europe. This is largely due to the success stories during the last five years.
A MULTI-ETHNIC CLINICAL RESEARCH ENVIRONMENT
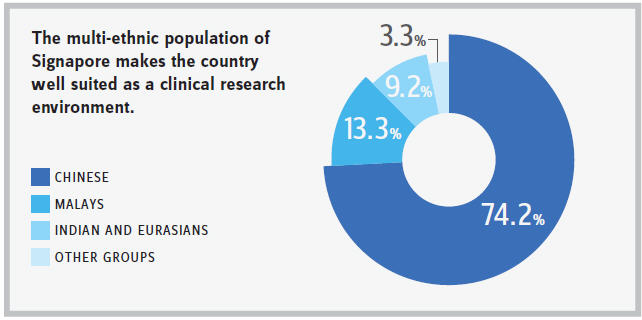
Singapore can provide an inimitable clinical research environment. The access to multi-ethnic populations is one of the unique advantages it can offer; the country’s population is composed of 74.2 percent Chinese, 13.3 percent Malays, 9.2 percent Indian and Eurasians, and other groups make up 3.3 percent of inhabitants. As such, it allows R&D companies to conduct multi-ethnic comparisons to establish the effects on different races, all in a single location — a location which the FDA and EMA (European Medicines Agency) will consider much more favorably than trials conducted in each of the countries of origin for those population groups. The drive by Singapore’s government to be a first-class clinical research destination has meant time has been spent ensuring the Health Sciences Authority of Singapore (HSA), the local regulatory body, has streamlined processes in place, thus allowing for faster approval timelines than not only the majority of other countries in the region but also globally. There is also a confidence in clinical trial data coming from Singapore, with leading GCP-trained investigators and sites, as well as an assurance that international quality standards are applied. The ability to rapidly recruit for these early-phase studies also ensures shorter trial timelines and, therefore, less overall expenditure.
Other developed countries in the region that have the infrastructure to conduct reliable Phase 1 studies, such as Korea, Taiwan, and Hong Kong, are less experienced in international clinical trials. Additionally for Phase 1 studies, more data needs to be provided, and timelines are longer than conducting later phase trials.
Singapore is central to the region and the hub of the life sciences industry, acting as a springboard to carry out further development across the rest of Asia as a candidate moves through the later phases. Conducting a Phase 1 study in Singapore can help an R&D company learn about the intricacies of performing development activities in Asia. In addition, choosing Singapore for these studies exposes a company to a new set of investors from a wealthy country.
WHERE TO CONDUCT PHASE 1 STUDIES IN SINGAPORE
There are three public independent units in Singapore where Phase 1 studies can be conducted, not including those for oncology studies:
- Changi General Hospital
- Sing Health Investigational Medical Unit
- National University Clinical Trials Unit
All of these units are very active and operate as extensions of the hospitals themselves, providing on-site support to investigators, as well as safety, security, and reassurance for study volunteers. In Singapore all units operate to the same stringent standards and efficiencies, with leading investigators at the helm and continued substantial investment from the government, for advanced infrastructure to support clinical trials. All of these factors make it easy to implement an early phase trial in the country.
The Clinical Trials and Research Unit (CTRU) at Changi General Hospital is run by highly experienced site personnel and provides the high level of medical sophistication essential for first-in-human studies. Since inception, the unit has conducted 39 industry-led Phase 1 studies. This unit specializes in pharmacokinetics, pharmacokinetics-pharmacodynamics, and pharmacogenetics studies, as well as studies on ethnic sensitivity testing, thorough QTc (corrected QT) and the effects of age and gender, drug-drug, and drug-food interactions.
CONSIDERATIONS FOR CONDUCTING PHASE 1 TRIALS IN SINGAPORE
- In Singapore, for first-in-human studies, the drug does not need to be approved in other countries before the study can be performed.
- For guidance on nonclinical and clinical requirements, Health Sciences Authority of Singapore (HSA) refers to applicable International Conference on Harmonization (ICH) guidelines and relevant guidelines issued by the major agencies such as the FDA and EMA.
- An export license is not required from HSA for shipping of biological samples overseas for testing.
- In general the documentation requirement to support FIH (first-inhuman) applications is not significantly different to that for later phase studies.
- If the investigational product is of a novel therapeutic class or mechanism of action, a presubmission meeting between the sponsor and regulatory authority can be arranged.
- Clinical trials materials imported into Singapore are exempted from goods and service tax of 7 percent.
- No fees are required for regulatory review or issue of a clinical trial certificate.
- English is the official language in Singapore. As English documents are sufficient for the initial submission dossier, a fast start-up time of four to six weeks is typical.
- Well-established, centralized drug depots are located in Singapore. Sponsors can utilize either direct shipment of IPs and trial materials from overseas to sites directly or have the materials stored at these depots for faster replenishment of materials at sites.

Dr. Christophe Tournerie is the founder and CEO of ClinActis Pte Ltd., a CRO that specializes in conducting clinical research in Asia Pacific.

Yew Lay Hwa is the assistant director of clinical trials & research unit at Changi General Hospital. She has extensive experience with clinical trial site management.
