What Patients Are Telling Sites About Trial Participation During COVID-19
By Nazneen Qureshi, director of patient engagement, LMC Manna Research

On March 11, 2020, COVID-19 was declared a global pandemic by the World Health Organization. Global economies shut down, countries were put on lockdown, and anxieties grew with the uncertainty of this new virus. In the realm of clinical research, sites started to receive communications from sponsors to hold enrollment for all trials and try their best to salvage what they could and retain their active participants. In this unprecedented time, the industry was trying its best to maintain the little control it had.
A week prior to WHO’s announcement, one of LMC Manna Research’s Ontario sites had experienced a COVID-positive patient, resulting in staff exposure and immediate site shutdown. Priorities immediately shifted, and the need for increased patient safety required an emergent change across all site SOPs. Two months later, these unforeseen circumstances allowed clinical research to propel in the direction we have been anticipating at our sites over the past five years: focusing on patient care through hybrid and virtual study execution and delivery.
With all these changes, the immediate concern recognized is how will COVID-19 impact our patients? How can we preserve patient retention?
At LMC Manna we understood that there is no better way to answer these questions than to ask our patients. Interviews were conducted with patients who are currently enrolled in a Phase 3 clinical trial, with questions focusing on the impact of COVID-19 during their trial participation. The average age of our respondents was 64 years old, participating in studies focusing on diabetes, lipid, or heart health research. (See Figure 1.)
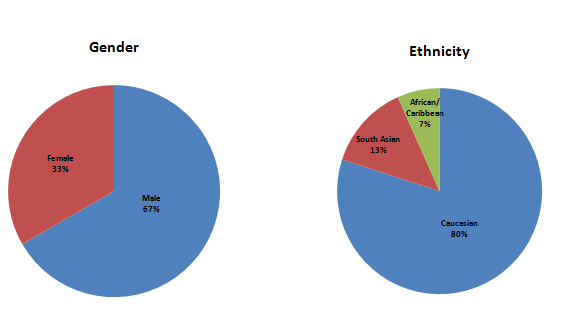
Figure 1: Demographic information
COVID-19 impacted our site-level processes and procedures, with updates made to mitigate any potential risk(s) our patients and employees could face. Studies are now being conducted in three forms: traditionally (with modifications), virtually, or as a hybrid of the two. For traditional study activities, our in-clinic protocol changed, requiring PPE to be used by both patients and staff. Clinics are maintaining physical distancing, including plexiglass barriers installed within each office, and the number of patients allowed inside each clinic at one time is restricted until further notice. In the virtual form of study participation, the trial visit is completely remote for the patient, with any required lab work completed by a designated staff member in the patient’s home. The hybrid study model pulls from the other two types: most of the patient’s visit is conducted over the phone or through videoconferencing (i.e., adverse event reporting, document completion), with patients required to visit the clinic only for essential labs and vitals. Direct to patient care (home visits) have been supplemented to ensure investigational product continuity and to perform essential vitals/labs when the patient is not able to visit the clinic.
Human Contact
When we asked our patients what form of study visit they preferred at this time, almost unanimously the answer was hybrid. Even with the impact of COVID-19, patients still want some form of human contact during their study participation. It was highly noted by survey respondents that the changes implemented by the site staff during in person visits had not impacted their desire to interact or be at the clinic: “I like seeing people.” “If I got a good mask, hand sanitizer, gloves, I don’t think there’s significant risk to myself.”
Technology Education
Though our respondent audience was older, an interesting point arose: When it came to fully virtual visits, patients had no problem using platforms like Zoom, if direction had been provided in advance. Though we usually resorted to the website instructions to educate patients about the platform, we realized we had to simplify the language further to better suit our average patient. In addition, there are individuals who do not have smartphones or computers, never mind access to any kind of video function. It’s important to consider and provide patients with the options that exist for them, rather than making the decision for them to proceed with fully remote visits. Patients want to have that choice.
Medication Delivery, Compassion, And Engagement
When asked how we could further support our patients, many appreciated medication deliveries and the option to have study visits in traditional, virtual, and hybrid forms, but what truly stuck out was the notion of “someone asking us if we are okay.” As many sites may have reduced workload, combined with the constant changes staff need to quickly adapt to, the element of simply checking in may have gotten lost during this critical time. Patient engagement is more important right now than it ever was before. While we try our best to provide monthly newsletters with updates, there are far more changes occurring, and at a pace so rapid, that it’s becoming difficult to keep all of our 2,000+ patients informed on a consistent basis.
Commitment To Participate
The biggest, and most remarkable, takeaway was that amid the changes COVID-19 has brought to our sites, patients are still committed to continuing their trials. Trial participation only became a concern for respondents if they were diagnosed with COVID-19. They are motivated to participate; they see the benefits to their health and that they are contributing to improving the health of others: “Medical advancement is based on studies, so you can’t expect medicines to advance without them, and if you can help, that’s great.”
How To Engage Your Patients
It is clear that our patients want to hear from us. Over 90 percent of our patients interviewed were interested in receiving some form of communication from their sites during this unprecedented time, and 71 percent preferred email as the primary form of contact. When asked how often they would prefer to receive updates, 46 percent preferred biweekly (see Figure 2). There were a variety of different ways patients wanted to stay engaged – updates on COVID-19, updates on their research trial, opportunities to ask questions about their trial, opportunities to discuss difficulties they are facing, etc. What was most important to them was “being informed, I can make better decisions” and “more awareness always is good – it provides hope.”
Our patients were also interested in “knowing what other people are going through.”. The most fascinating finding was that 50 percent of the patients wanted to communicate with others who were also taking part in research trials! They wanted to share experiences within this unique research community.
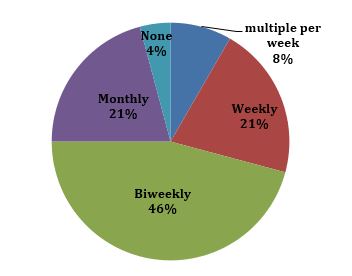
Figure 2: How often would you like to be contacted?
Conclusion
The insights our patients provided were invaluable. As we continue to adjust to our new norm with COVID-19, we need to continue focusing on patient engagement. Though a patient’s reason to leave a trial is most likely not due to COVID-19, inconsistencies in communication during these difficult times may be the reason instead. In an environment so volatile, we need to keep our patients educated on the matter of COVID-19 and their health – they will want to hear it from us.
Sites are already overwhelmed with adopting new SOPs and handling patient safety. As an industry, we can engage patients in a unified, consistent manner to streamline communications and reduce site burden. For example, TrialScout (patient rating and review platform for clinical trials) has established a 12-week enhanced patient engagement program developed to address the new challenges during the COVID-19 pandemic. The program allows for a two-way communication between sites and patients for process updates, community based forums, frequently asked questions, as well as light hearted content and good news.
The most important thing that you can do right now is to stay engaged with participants in a two way conversation that fosters trust, transparency and respect. Help your site achieve trial continuity the best possible retention rates through patient engagement!
About The Author:
 Nazneen Qureshi is the director of patient engagement at LMC Manna Research, an integrated clinical research site organization with 21 active research sites in Canada. In this role, she manages study start-up activities, focusing on recruitment and retention strategies. Her objective is to increase the understanding of patient perceptions to improve clinical research processes such as study design, experience, and motivation to participate. Qureshi is the recipient of the SCRS 2017 Site Patient Recruitment Innovation Achievement Award. She holds an HBSc in neuroscience and psychology from the University of Toronto and is completing her MBA at the Lazaridis School of Business and Economics. You can reach her at nazneen.qureshi@lmcmanna.com.
Nazneen Qureshi is the director of patient engagement at LMC Manna Research, an integrated clinical research site organization with 21 active research sites in Canada. In this role, she manages study start-up activities, focusing on recruitment and retention strategies. Her objective is to increase the understanding of patient perceptions to improve clinical research processes such as study design, experience, and motivation to participate. Qureshi is the recipient of the SCRS 2017 Site Patient Recruitment Innovation Achievement Award. She holds an HBSc in neuroscience and psychology from the University of Toronto and is completing her MBA at the Lazaridis School of Business and Economics. You can reach her at nazneen.qureshi@lmcmanna.com.
