A Look At 2015 Outsourcing Trends And What To Expect In 2016
By Nigel Walker, Managing Director, That’s Nice
This has been another exciting year in outsourcing, as mergers, acquisitions, and other collaborations continued with CROs and CMOs moving toward functioning as strategic partners.
 indeed, while the concept has been around for some time now, the reference to contract development and manufacturing organizations (CDMOs) is more prevalent when it comes to defining the strategic partner approach. The continued expansion of organizations such as Patheon speaks to the trend.
indeed, while the concept has been around for some time now, the reference to contract development and manufacturing organizations (CDMOs) is more prevalent when it comes to defining the strategic partner approach. The continued expansion of organizations such as Patheon speaks to the trend.
There has been further consolidation with CROs as well, with LabCorp’s acquisition of Covance and Chiltern’s acquisition of Theorem Clinical Research. Similarly, Big Pharma companies look toward global CROs to share operational practices. It will be interesting to see whether these consolidated companies, both CROs and CDMOs, are able to fully integrate their services and improve their performance
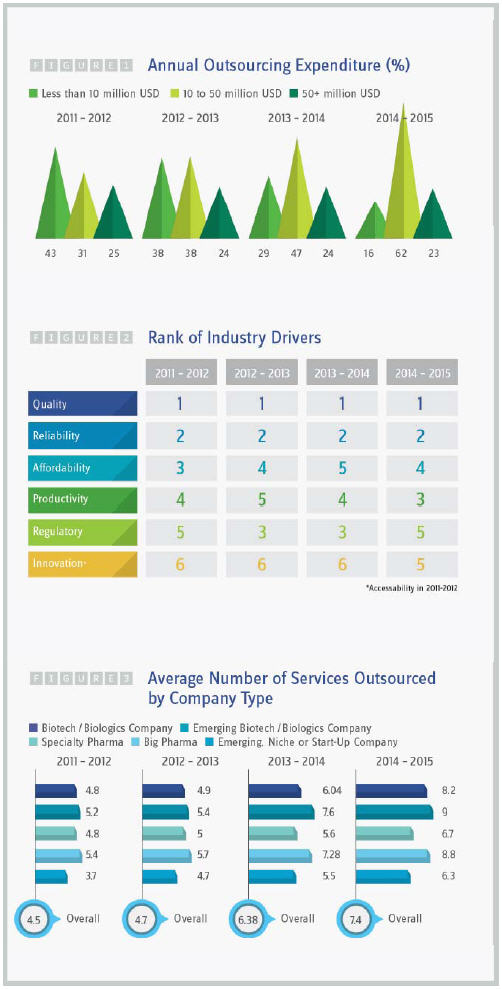
According to the Nice Insight report on 2015 outsourcing trends, this year saw another big jump in expenditure for CROs and CMOs, maintaining the continuously escalating outsourcing spend over the past four years. Nearly two-thirds (62 percent) of survey respondents from pharmaceutical and biotech companies spent $10 million to $50 million USD in 2014-2015 for outsourcing, a jump of 24 percent from last year and double the number of companies who spent this amount in 2011-2012. Significantly fewer companies (16 percent) spent less than $10 million, a drop from 29 percent last year and 43 percent in 2011-2012 (Figure 1).
The demands from industry service providers have never been greater. Some key trends driving the continuously rising outsourcing budgets include a growing pipeline of biologics, complex therapies and delivery systems, and precision-based medicines, as well as larger, more complex clinical trials, real-world evidence studies, and the need for sophisticated new technologies, all of which require advanced, integrated expertise. Health-economics and outcomes research and data analytics services are also in demand. Biopharmaceutical and biologics companies are increasingly turning to service providers for all aspects of drug development, partly to avoid the very high capital expenditure and long lead times needed to construct, equip, and validate manufacturing facilities.
Outsourcing is an efficient, costeffective way to meet these rapidly changing industry needs. With cost and regulatory pressures on drug manufacturers continuing to increase, it is not surprising that growth of the global CRO and CMO market is healthy at 9 percent and 6.4 percent CAGR, respectively. The globalization of clinical trials also reflects the growth of healthcare and drugs that treat acute conditions for unique patient populations (e.g., orphan drugs). Personalized medicine and advances in cell and gene therapy also require new research and manufacturing services.
The continued increase in expenditures coincides with a decrease in the prioritization of affordability as one of the drivers for selecting an outsourcing partner. While quality and reliability remain the leading factors influencing the choice of partner, regulatory compliance was less important this year, dropping to the bottom of the qualification list of six factors and tied in ranking with innovation (Figure 2). Other considerations include technical capabilities and on-time delivery.
As for the number of services outsourced by company type, Nice Insight research data showed that the average number of services outsourced has increased across the board (Figure 3).
Survey Methodology: The Nice Insight Pharmaceutical and Biotechnology Survey is deployed to outsourcingfacing pharmaceutical and biotechnology executives on an annual basis. The 2014-2015 report includes responses from 2,303 participants. The survey is composed of 240+ questions and randomly presents ~35 questions to each respondent in order to collect baseline information with respect to customer awareness and customer perceptions of the top ~125 CMOs and ~75 CROs servicing the drug development cycle. Five levels of awareness, from “I’ve never heard of them” to “I’ve worked with them” factor into the overall customer awareness score. The customer perception score is based on six drivers in outsourcing: Quality, Innovation, Regulatory Track Record, Affordability, Productivity, and Reliability. In addition to measuring customer awareness and perception information on specifi c companies, the survey collects data on general outsourcing practices and preferences as well as barriers to strategic partnerships among buyers of outsourced services.
If you want to learn more about the report or how to participate, please contact Nigel Walker, managing director at Nice Insight, by sending an email to nigel@thatsnice.com.
