Essential Elements Of Technology Transfer
By Peter Alexander and Phillip Ramsey
The life cycle of pharmaceutical development always requires the transfer of the production process and analytical methods from development into GMP manufacturing and quality control laboratories. This transfer can take many forms, from traditional pharmaceutical organizations with full in-house product development and manufacturing capability to small startup organizations using contract development and manufacturing organizations (CDMOs) to bring their discoveries from the research bench to human clinical trials. Regardless of the nature and maturity of the organization, an effective technology transfer program is essential to the success of pharmaceutical development.
An effective technology transfer process is well documented and results in a seamless transfer of information and materials from the sending organization or sending unit (SU) to the receiving organization or receiving unit (RU), culminating in successfully executed test methods and production processes. Technology transfer programs provide clear and concise guidelines for the sending and receiving organizations in order to ensure that the transferred materials and methods generate suitably comparable results. They also help to ensure both timely success of the transfer process and manufacturing of a comparable product that meets all necessary critical quality attributes (CQAs) and regulatory requirements.
Such programs include knowledge and materials transfer, training runs, document development, and comprehensive review of data generated during the transfer process. A complete understanding of the project’s goals, execution methods, and product acceptance criteria are realized when open, direct, and well-managed communication lines are created and maintained between the SU and RU.
These principles apply equally to small, virtual organizations that are sending a process to a CDMO and large, fully integrated pharmaceutical companies that are developing and manufacturing their own products. Likewise, these principles apply at all stages in the pharmaceutical development life cycle, from the discovery stage through clinical development and market launch to post-marketing process improvement changes.
Technology Transfer Categories
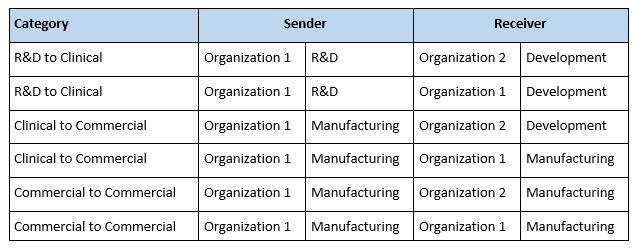
Different categories of technology transfer can be defined by the nature of the SU and RU (Table 1). Technology transfer is often thought of as the external type in which a drug development or research company transfers a process and its analytical methods to a CDMO. Alternatively, the drug development organization might be a business unit within a larger company that has its own GMP manufacturing capability, thus making the transfer internal. Table 1 illustrates the transfer process schematically. It may also occur that a drug product made in one GMP facility needs to be manufactured elsewhere as a GMP product, either at a different CDMO (an external transfer) or at a second manufacturing site within the same company (an internal transfer).
Table 1. Example Technology Transfer Categories
Essential Elements Of Technology Transfer
Technology transfer programs are composed of both knowledge and materials transfers and are subject to regulatory guidance.1 Knowledge transfers involve both written documentation and oral communications. Oral knowledge transfer takes the form of meetings between subject matter experts (SMEs) from the SU and RU, in-person observation during method execution, and method and process training programs.
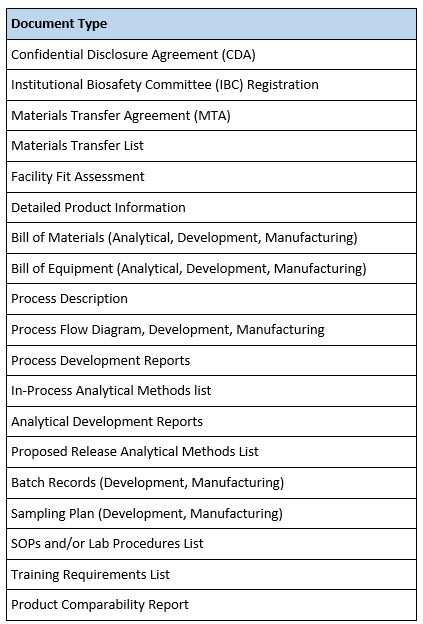
Written documentation (Table 2) includes common and not-so-common documents. For example, some organizations may not require an Institutional Biosafety Committee (IBC) review of a process prior to initiating work with a new biological agent. Even if an IBC is not required, it is advisable that the receiving organization be fully apprised of the safety risks associated with an incoming process or analytical method and the materials used to execute them. Thus, a full biological, chemical, and occupational safety assessment should be part of a thorough technology transfer program.
Confidential disclosure agreements (CDAs), materials transfer agreements (MTAs), and quality agreements are generally required for transfers between different organizations, whereas internal transfers would not typically require these documents.
Table 2. Technology Transfer Documents
Materials Transfer Agreements
In most cases, an SU will need to provide certain materials to the RU for the receiver to produce and test the drug product. Transferred materials might include cell stocks, reference standards of the drug product for comparison studies, specialized analytical methods reagents (e.g., antibodies for product detection and quantitation), or specialized materials required for manufacturing that would not otherwise be commercially available. Prior to sending these materials, an MTA should be in place that describes the conditions and limitations under which the receiving organization may use the sender’s materials.
Facility Fit Assessments
One of the first steps in the technology transfer process is an assessment of the facility fit at the receiving organization. This involves a review of the room layouts and utility requirements for the incoming methods, as well as the associated equipment requirements. Equipment suitability for the intended process should be assessed at this stage. Validation of new GMP equipment may be required. For GMP manufacturing facilities, the fit assessment should also include room classifications along with materials, personnel, and equipment flows through the facility. If these criteria are not met, then new room configurations, utilities, equipment, or flows may be required. In this case, the transfer may not even occur if the receiving organization cannot make the necessary adjustments.
Detailed Product Information
Another early-stage transfer requirement is the transfer of a detailed product information package. This package, which is often available from the product sponsor as the Target Product Profile (TPP), should include name and target indication of the drug substance, its molecular weight, isoelectric point (protein products), glycoforms (protein products), sequence data (DNA, RNA, or amino acid), stability, and formulation, fill, and finish requirements. As noted above, this information will be important for the safety assessment, and for developing an understanding of the product at the RU.
Process-related Documentation
Process-related documents are essential for developing a complete understanding of how a product is to be manufactured and why those particular process parameters were selected. A similar package of documentation should be assembled for assay method transfers. Process flow diagrams (PFDs), process descriptions, a bill of materials (BoM), batch production records, and lab procedures are all part of a process transfer documentation package.
It is important that the BoM used at the research and development stage be reviewed for compatibility with cGMP requirements. Many research-grade processes do not use GMP-grade raw materials and supplies, which in some cases necessitates a material replacement study. Most often, research-grade materials and supplies can easily be changed to compendial grade substitutes (e.g., USP, NF, FCC, or multi-compendial grade). This may be less important for materials and supplies to be used in analytical development and quality control, but it is essential for GMP manufacturing.
Similarly, a bill of equipment should be used to assess each of the critical equipment requirements in transferring analytical and process methods. Equipment used in GMP manufacturing must be validated through installation (IQ), operational (OQ), and performance qualification (PQ). It is essential that the equipment used in development be scalable to the projected GMP manufacturing scale and therefore it is advisable that the development equipment used during technology transfers be validatable, if not actually validated.
Process description documents can take various forms but must include detailed information regarding each process unit operation, including process inputs (e.g., materials, supplies, process intermediates), critical process parameters (CPPs) (e.g., temperature, pressure, pH), and process outputs (e.g., sampling points, in-process data outputs). Much of this information is included in PFDs, which can be appended to a process description narrative. The process description narrative is often a summary of the final methods and conclusions drawn from analytical and process development reports.
A comprehensive list of in-process and proposed product release methods is another essential element of the technology transfer package. This list is used by the RU to create sampling plans for the incoming process and to establish which analytical methods are required at which steps in the process. Further, the proposed release methods list identifies those analytical methods that must be qualified (or validated) for use in GMP batch analysis.
Although not common in many R&D settings, any written laboratory procedures or SOPs should be transferred from the SU to the RU. Often analytical and process development units at CDMOs will use written procedures, including lab protocols, SOPs, and development batch records, to document their work. These documents are extremely useful when transferring a method from development to manufacturing.
Training Requirements
Given that an incoming process may be different from processes already in place at the RU, a list of training requirements essential to the accurate execution of its analytical and process methods is an important document to consider. Training for a new process may include training on new equipment and on new analytical and process methods or unit operations. Such training may include reading new SOPs and batch records, observing the execution of the new method at the SU or in the development laboratory at the RU, and hands-on training to verify competence in executing the method. GMP training programs at the RU may need to be updated to accommodate these new training requirements.
Product Comparability
A successful technology transfer will result in the production of a drug product at the receiving organization that meets or exceeds the product specifications for each CQA of the product at the SU. Having suitable reference material from the SU is essential for this analysis. After executing the technology transfer batch(es), the analytical team at the RU will assess the biological and physical quality attributes of the product in comparison with the reference standard.
Technology Transfer Criteria
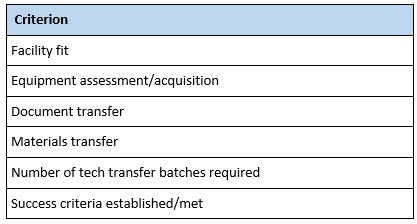
A number of criteria can be used to track the progress and success of a technology transfer program (Table 3). One of the first criteria to establish is the number of successful assays or process batches to be executed. Without establishing this prospectively, the RU can be left with an open-ended, never-ending project.
The same is true of success criteria themselves. A prospective set of acceptance criteria should be established before technology transfer batches are initiated at the RU. Failing to do so may result in having to execute numerous transfer batches to meet criteria goalposts that are continuously moved by the SU.
Table 3. Technology Transfer Checklist
Regulatory Considerations
Khedir and Bouslah recently summarized some key regulatory aspects of an effective technology transfer program.2 These authors emphasize the importance of project planning and of executing a formal gap analysis and a formal risk assessment of the transfer program.2 They cite guidelines from the World Health Organization that describe the approaches to be used for these assessments, some of which are described here.
Conclusion
A well-planned and comprehensive technology transfer program is the best way to avoid surprises when transferring analytical and process between manufacturing sites. Combining the tools described here with thorough risk assessments will help ensure a smooth and effective transfer resulting in production of a product that meets or exceeds its original product specifications.
References
- International Council for Harmonization (2008). Quality Guideline Q10: Pharmaceutical Quality Systems. ICH, Geneva
- Khedir and Bouslah (2023). Navigating regulatory guidelines for effective tech transfer. Bioprocess Online, June 22, 2023
Additional Reading:
- Gallo, D. et al (2022). Technology Transfer: Technical Report No. 65 (Revised 2022). Parenteral Drug Association.
- USP<1224> Transfer of Analytical Procedures
About the Authors:
 Peter Alexander has over 35 years of industrial biotechnology experience in process development for therapeutic and vaccine products in numerous technology platforms, including conjugate polysaccharides, recombinant proteins, DNA vaccines, adenovectors, and virus-like particles. He is an independent consultant with a focus on CMC, technology transfer, and scale-up. He has held management and senior director positions for Aeras, IDT Biologika Corp., Lonza, Cambrex, and Baxter, among other companies.
Peter Alexander has over 35 years of industrial biotechnology experience in process development for therapeutic and vaccine products in numerous technology platforms, including conjugate polysaccharides, recombinant proteins, DNA vaccines, adenovectors, and virus-like particles. He is an independent consultant with a focus on CMC, technology transfer, and scale-up. He has held management and senior director positions for Aeras, IDT Biologika Corp., Lonza, Cambrex, and Baxter, among other companies.
 Phillip Ramsey is senior vice president of technical operations at Sangamo where he oversees manufacturing, technical development, supply chain, and quality. He was previously the vice president of technical development overseeing process and analytical development, vector delivery, and CMC program management. Prior to Sangamo, he worked at Emergent BioSolutions most recently as senior director in the biodefense division. Before that, he was at Leidos where he participated in the design and construction of a 130,000-square-foot vaccine pilot plant for the NIH. He has contributed to multiple regulatory submissions for product approval and more than 50 investigational new drug submissions. He received a BS in chemistry from the University of Nebraska, an MS in biochemistry from John Hopkins University, and an MBA from San Diego State University.
Phillip Ramsey is senior vice president of technical operations at Sangamo where he oversees manufacturing, technical development, supply chain, and quality. He was previously the vice president of technical development overseeing process and analytical development, vector delivery, and CMC program management. Prior to Sangamo, he worked at Emergent BioSolutions most recently as senior director in the biodefense division. Before that, he was at Leidos where he participated in the design and construction of a 130,000-square-foot vaccine pilot plant for the NIH. He has contributed to multiple regulatory submissions for product approval and more than 50 investigational new drug submissions. He received a BS in chemistry from the University of Nebraska, an MS in biochemistry from John Hopkins University, and an MBA from San Diego State University.
