eTMFs: Moving From Electronic Filing Cabinet To Strategic Asset
By Jennifer Goldsmith, VP of Veeva Vault, Veeva Systems and Lisa Mulcahy, owner and principal, Mulcahy Consulting
In 2010, McKinsey & Company published a report on the need to reinvent drug development through technologies designed to streamline the clinical trials process. The report recommends implementing technologies that represent a “clean-sheet” or redesigned traditional clinical trial methodology.
 McKinsey identified having a single document repository with workflow management and the ability to track cost, quality, and speed as a core factor for business transformation. Turns out, McKinsey was onto something.
McKinsey identified having a single document repository with workflow management and the ability to track cost, quality, and speed as a core factor for business transformation. Turns out, McKinsey was onto something.
The growing functionality in electronic trial master file (eTMF) applications enables life sciences companies to streamline many inefficient processes that can slow clinical trials. Moreover, today’s eTMFs enable sponsors and CROs to better track a study’s progress by tracking the status and completion of critical documents. Doing so enables both types of organizations to proactively identify operational challenges and avoid costly delays. In fact, advanced eTMF applications can become a crucial source of trial information and performance insights to help improve and speed clinical development.
The eTMF — the electronic compilation of documents and other content that chronicle the conduct of a clinical trial — is gaining traction. According to the 2012 TMF Reference Model survey, 27 percent of respondents claim to be actively building or evaluating an eTMF, up from 17 percent in 2010. TMF management has evolved, too, from paper-based files to electronic “filing cabinets” of scanned documents and, today, to purpose-built applications, some of which have even moved to the cloud. Unlike their predecessors, modern eTMF applications provide visibility into trial operations and help ensure that the TMF is always inspection-ready. The wealth of information these applications collect about a study’s start-up, ongoing operations, and close-out allows the eTMF to function as a business planning tool.
Widespread industry research highlights how document-centric processes directly impact major benchmarks, such as study start-up and close-out. Paper-intensive processes, such as contract negotiations and ethics committee approvals, are top causes of study delay, suggests data from a 2011 global CenterWatch study. Furthermore, a collaborative study on trial start-up conducted by the Tufts Center for the Study of Drug Development reveals that, on average, a Phase 2/3 study takes 16.7 months from protocol approval to 100 percent approved sites initiated. Within this time frame, high volumes of paperwork tied to pre-study visits, site selection, contract negotiations, site initiation, and first-patient visits are generated. And, according to Veeva Systems’ 2014 survey of TMF owners (n = 260), 63 percent of respondents say paperless study and site start-up processes would significantly shorten clinical development times.
Recognizing the efficiencies that an eTMF application offers an organization is one thing. Transforming an eTMF into a truly strategic asset capable of improving the bottom line is another matter. To extract the full potential of an eTMF, life sciences organizations must take a few important steps with the partner, the application itself, and their own organization. These include:
- Step 1 – Define the collaborative process among internal and external partners.
- Step 2 – Build a repeatable framework, outlining what documents are expected, what they are called, and who is responsible.
- Step 3 – Leverage performance metrics involving study-related documents to provide visibility and early problem resolution.
DEFINING A COLLABORATIVE PROCESS
The growing number of trial stakeholders (CROs, trial sites, agencies, committees, patients) has dramatically increased the complexity of assembling the numerous pieces of the TMF into a coherent package. During the past decade, sponsors have been attempting to improve overall trial efficiency by concentrating operational efforts on fewer but more strategic CRO partners. In fact, 65 percent of sponsors are now using fewer than five CROs, according to Vantage Partners’ Sponsor- CRO Collaborative Study. CenterWatch research from 2013 shows that 87 percent of the top 30 pharmaceutical companies have at least one strategic functional service provider (FSP) or multi-FSP alliance — collaboration that will clearly play a pivotal role in their success or failure.
With fewer partners, it is easier to specify which one is responsible for which elements of the information and which SOPs will be used. When partners run multiple trials together, both benefit from using a single process to manage their collaboration. A shared system for collaboration results in efficiencies in trial execution, which once defined, can be reused from study to study.
In many cases today, however, the sponsor and/or CRO maintain an eTMF on their own network, blocking access to outsiders. In this scenario, stakeholders send documents via paper shipments or email and maintain separate copies of TMF documents that need to be reconciled at the conclusion of the trial. Alternately, a cloud-based eTMF is by its very nature easily and securely accessible by all parties. Sponsors can define new processes that are more efficient up front, maintain visibility throughout the trial, and help ensure the TMF remains inspection-ready at all times. This type of collaborative and open process begins by uploading a document into a cloud eTMF. Because all parties have direct access, physical distribution of content becomes obsolete, eliminating the need for emailing copies of documents as attachments. Renee Fate, senior manager of document management at Kythera Biopharmaceuticals, describes one process the company is redefining in an initiative to take full advantage of its new cloud TMF system. “We partnered with a CRO for both regulatory and clinical support, and their SOPs had both teams sending us the same document. Next time, we’ll define one process that has their clinical team uploading documents into our cloud TMF where I can review them before sending the approved documents to both of our regulatory teams.”
Managing collaborative processes within the eTMF combines information exchange and tracking into a single system. Not only does collecting TMF documents become more efficient, but also all parties gain visibility into status and outstanding tasks.
“We are shaving at least 40 percent off the amount of time needed to reconcile the TMF at the conclusion of a trial with our cloud system,” added Fate. “Now, we larghave full visibility and can track the status of the TMF in real time for the duration of the study so we can identify bottlenecks or missing documents along the way. We don’t have to wait until the end when ‘surprises’ can force us to backtrack, which wastes so much time. We can now manage workflow much better and close studies sooner, which will translate into cost savings.”
BUILDING A REPEATABLE FRAMEWORK
Building a repeatable TMF framework involves defining expectations upfront to ensure all TMF participants are aligned and in agreement on what the TMF artifacts are called, when they are due, and who is responsible for filing them. In order to know what content is missing or late, all contributors must first understand what is expected. A repeatable framework sets expectations at the outset, reinforces the collaborative process, and improves overall efficiency.
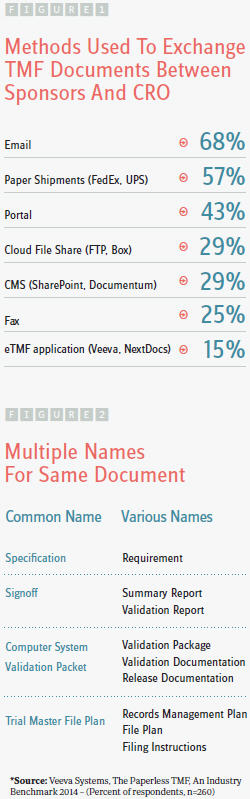
Standardizing a common nomenclature drives better communication by harmonizing the filing efforts of diverse stakeholders. When multiple parties refer to items by different names (Figure 2), filing and tracking become confusing, increasing the chance for error. The nomenclature defined by the Drug Information Association’s (DIA’s) TMF Reference Model represents input from hundreds of pharmaceutical companies, CROs, regulatory agencies, and vendors from across the globe. In addition to standardized naming, the TMF Reference Model introduces standards for content, structure, and metadata. For these reasons, more and more clinical trial sponsors, including Kythera, are leveraging this model to build their own repeatable framework.
When setting the framework, it is essential to establish time frames for completing management milestones, as well as roles and responsibilities for execution. In many cases, the responsibility for filing TMF documents and other content will shift from a records management function to the author/owner in the TMF. Managing a successful process change is critical for gaining many of the benefits associated with using an eTMF. Because of this, establishing a repeatable framework is an important part of the change management process. Defining each stakeholder’s role is also critical to successful outsourcing, finds an Avoca Group survey of 237 respondents. Collaborative relationships “require absolute clarity in roles and responsibilities and up-front planning assumptions,” Avoca states. Typical clinical collaborations have lacked this clarity, sometimes resulting in difficulties and disappointment in the relationship.
“The biggest issue when it comes to transitioning to a new type of system – from paper to electronic, for example – is the fear of losing control. But, when employees and partners see the increase in efficiency that comes from a more streamlined, repeatable process, then they are more likely to embrace the system and accept a new ‘digital’ mindset,” said Fate.
Additional elements of the repeatable framework include operationalizing SOPs by configuring them within the eTMF application, essentially codifying them into system workflows. The eTMF application orchestrates task completion across companies and stakeholders, in keeping with company SOPs. A common workflow automates many manual steps, improving productivity and trial efficiency. By comparison, a paper-based TMF or eArchive relies on people remembering and following written SOPs and then documenting them.
When collaborative processes are coupled with a repeatable framework, the foundation is in place to begin defining and leveraging performance metrics.
DEFINING AND TRACKING PERFORMANCE METRICS
 The eTMF can track operational metrics for a specific study by which documents have been completed or remain unfinished and which need follow-up. These simple daily metrics are a good place to start, according to Linda Sullivan, COO of Metrics Champion Consortium, an association dedicated to standardized performance metrics.
The eTMF can track operational metrics for a specific study by which documents have been completed or remain unfinished and which need follow-up. These simple daily metrics are a good place to start, according to Linda Sullivan, COO of Metrics Champion Consortium, an association dedicated to standardized performance metrics.
A 2014 survey by NextDocs supports the notion that operational metrics are important but remain a challenge. In fact, the survey indicates that the second largest challenge in managing a TMF is the lack of visibility into the status of clinical trial documentation. eTMF reports such as study site document status, site acknowledgement of investigator brochure, and document expiry can all help inspection readiness by providing greater visibility into what’s approved and what’s missing.
These common, trial-specific performance metrics — efficiency and completeness — establish a baseline for improvement, allowing managers to look at metrics in an organized way as opposed to extrapolating from paper-based processes. However, as more data is collected over time and across multiple trials, it also becomes possible to identify trends. “What about improving cycle time? Time to database lock? Are things getting better? Worse? Which sites are the best performers? When you start to ask those questions and get answers, users are ready to move toward a more mature phase in the process,” says Sullivan. “Eventually, the eTMF expands in value when organizations can determine whether problems are unique to one study or if there is a common problem across multiple trials. For example, if the contracting process is too lengthy for numerous trials, what steps can be taken to shorten this activity and improve cycle time?”
This is the point at which the eTMF builds to a greater level of sophistication. The eTMF can help with business decisions by gathering an array of quality, performance, and operational metrics that are both internal and external and across multiple sites and studies.
eTMF: AN ESSENTIAL TOOL
With increasing pressure to meet clinical trial timelines and rein in costs, sponsors and CROs are looking to the eTMF as an essential tool for completing and collecting the array of documents involved in clinical trials — and for using the resulting data to identify process improvements. The urgent need for greater visibility into study conduct and quality benchmarks for trial operations is driving the industry’s growing use of new technology. This evolution toward a single source of shared electronic documents is helping stakeholders modify processes that improve collaboration and gain business insights.
“We recognized from the outset that going electronic with our TMF would be critical to improving efficiency and enabling seamless collaboration with trial stakeholders around the world. Now, in order to maximize the system’s value, we are reengineering our SOPs to reflect the advantages of a cloud-based eTMF and to move forward with study success — ultimately delivering much-needed drug therapies to patients faster,” concluded Fate.
