How Efficient Is The Toyota Production System In Biopharma?
By Alain Pralong, CEO, Pharma-Consulting ENABLE GmbH
 The Toyota production system (TPS) — originally designed for the automobile industry to reduce waste so companies could become economically competitive — recently has become more popular within the biopharmaceutical industry for managing the complex endeavor of new product development and manufacturing. However, there are those who would question the effectiveness of this management approach for life science executives.
The Toyota production system (TPS) — originally designed for the automobile industry to reduce waste so companies could become economically competitive — recently has become more popular within the biopharmaceutical industry for managing the complex endeavor of new product development and manufacturing. However, there are those who would question the effectiveness of this management approach for life science executives.
I have developed successful Lean Six Sigma approaches for product development value streams (PDVS) and technical life cycle management value streams (TLCVS). In addition, I have developed, embedded, and used TPS elements, so I would like to share my experiences and highlight the benefits and limitations.
A PROCESS THAT FOSTERS BETTER DECISION MAKING
The biopharmaceutical industry is characterized by two particular hallmarks: long development times for bringing a new drug to market and long drug life cycles. For example, it takes 12 to 15 years to develop a new vaccine that then needs to be supplied to the market for decades. Both of these factors need to be taken into consideration when implementing TPS concepts in this industry. To do so, you should view product development and technical life cycle management as a continuum (see Figure 1). This continuum ensures reliable product supply and transfers experience from production to product development and maintenance.
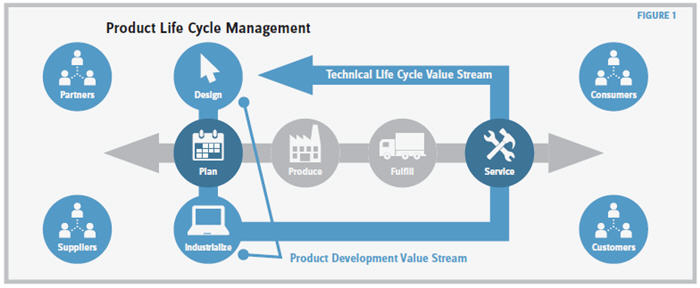
A Kaizen-driven (i.e., continuous improvement) value stream organized and governed through stage gates (similar to milestones) helps all process contributors understand what to deliver, by when, and at what level of quality without major project management effort. This process fosters educated decision making regarding drug development depending on the status of the deliverables and the results at a given stage gate. Furthermore, it eliminates the risk of forgetting or not executing activities related to the required quality level since the CMC (chemistry, manufacturing, and controls) documentation is built over time instead of compiling it just before the BLA (biologics license application) submission.
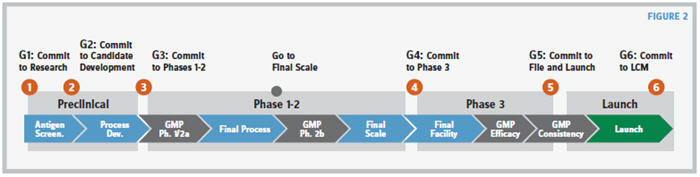
The major impediments to the success of this approach include the willingness (i.e., maturity level) of the organization to respect a value stream and the organization’s efforts toward compiling the documentation needed to enable educated and risk-mitigated decision making. Time, perseverance, continuity, and a first positive experience are required to motivate and persuade personnel to adhere to the principles of a TPS-inspired value stream for new product development. Embedding the PDVS, even on products in late-stage development, has permitted identification and timely resolution of gaps that could have the potential of delaying BLA submission and product launch. Figure 2 presents an example of how a PDVS can be organized and structured.
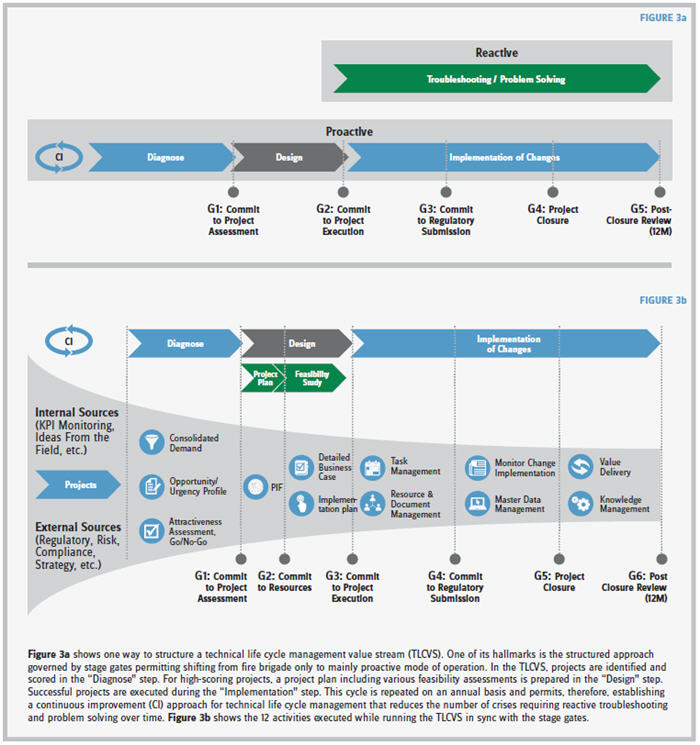
MOVING AWAY FROM A REACTIVE APPROACH
It’s interesting to me that the biopharmaceutical industry traditionally has pursued the dogma of “the process is the product,” which has resulted in a mentality of complete risk aversion coupled with more reactive rather than proactive strategies. But today’s biopharmaceutical companies need to be able to identify risks and opportunities within a process and a product in a proactive way. To do so, a risk/reward matrix has to be prepared that reviews four main areas: business risk/opportunity, urgency, benefit, and cost of implementation. This new way of working establishes educated and balanced risk/reward management. Obviously, the complexity of this risk/ reward matrix management is multiplied significantly with the number of different processes and products in the portfolio. Vaccine manufacturers, for example, can have over 30 marketed products. Furthermore, the risk/reward matrix needs to be continuously updated and in sync with the expectations of the regulatory authorities.
In my experience, I found developing a TLCVS with distinct stage gates to be very effective in transitioning a company to be more proactive in its thinking regarding drug development. All risks and opportunities for a given process and product are collected and then scored against an established rating system consisting of 11 questions. For each question a set of five answers is predefined to enable “answers that use the same yardstick.” The outcome of the scoring creates the risk/reward matrix.
Applying the TLCVS gives you heightened awareness regarding potential risks and rewards, which, in turn, enables you to proactively mitigate/capture those risks and rewards. As for the PDVS, the major limitations reside in the maturity level of the organization. You not only have to identify risks and rewards, but also implement appropriate follow-up actions regarding your findings.
Of course, this kind of proactive risk mitigation and reward capturing comes at a certain cost. That’s probably why some organizations choose to postpone proactive projects such as this in favor of saving money in the short term. But that’s a dangerous strategy considering recent regulatory enforcement actions that show the cost of dealing with an average warning letter can approach $100 million.
YOU HAVE TO BE WILLING TO ADAPT
Through my work in product development and technical life cycle management, I had the chance to develop and embed TPS approaches at two biopharma companies. In the beginning of these projects, personnel were often skeptical of TPS since it originated in car and chip manufacturing. But once they witnessed its benefits, and were able to shed the mentality that “the biopharmaceutical industry is different,” they became supporters of this new approach.
TPS in the biopharmaceutical industry brings product development and maintenance to a new, currently unmatched maturity level that can cope with strong pipelines and portfolios. When using TPS in the biopharmaceutical industry you have to adapt your review and intervention cycles to the requirements of drug development and maintenance. Then, the status of the deliverables for each stage gate determines management’s decision-making options.
HOW BIOPHARMA SENIOR MANAGEMENT CAN BEST UTILIZE TPS ELEMENTS
In my experiences, there are certain elements of TPS that biopharma senior management find very useful. For example, dashboards that display KPIs (key performance indicators) are a TPS element that is very popular at the senior management level. Indeed, knowing the status of the different KPIs (green, amber, and red) is important for determining the overall state and performance of an organization. However, some people question if this is the right approach for decision making at the senior management level. In contrast to the TPS decisionmaking points, which are in sync with the stage gates of the PDVS and TLCVS, these dashboards show lagging indicators since data extraction and preparation takes significant time. Furthermore, to reduce the number of KPIs, often various parameters are absorbed and integrated, making it difficult to understand the driver for the shown performance. Often, a further specific deep-dive is required to obtain a comprehensive overview of the situation leading to an amber or red KPI. Normally, such a deep-dive requires another two to three months to ascertain the details. Even worse, the ability to make relevant and appropriate decisions is limited since senior management level is far away from the personnel on the shop floor creating the data used to generate the KPIs. Hence, decisions come too late or are not appropriate anymore for the evolving situation on the shop floor.
To me, there’s no doubt that TPS is a useful and efficient tool for the biopharmaceutical industry. But transitioning to this more proactive way of thinking — which can be applied throughout an organization — requires a shift in the way senior management gathers and assesses its data for decision making.
