RespireRx: Life Science Leadership In Action
By Wayne Koberstein, Executive Editor, Life Science Leader
Follow Me On Twitter @WayneKoberstein
The Enterprisers: Life Science Leadership In Action

Suffocation seldom gets the credit it deserves for causing death in so many conditions — from sleep apnea to heart failure to drug overdose. But if you view the large variety of those cases at a higher resolution, you will see “respiratory failure” as the common final, fatal effect. You don’t need a pillow held over your head to suffocate; the cause can be anything, any disease or condition, that keeps your lungs from breathing adequately. How many lives could be saved by a drug that reversed respiratory suppression in its various settings? It is a long story, and we’ll have to wait still longer to see its ultimate outcome, but one enterprising company, RespireRx Pharmaceuticals, appears to be making headway toward that goal.
 At the BIO meeting in June, company chairman and chief scientific officer Arnold Lippa, Ph.D., told me the long history of RespireRx, known as Cortex until mid-December 2015. It is a tale of persistence, and even audacity, through repeated setbacks and recoveries. The company started up almost four decades ago atop a wave of enthusiasm for ampakines, a class of compounds that enable glutamate receptors to stimulate neural activity in the brain. At the high point of the wave, however, reverses in the field curtailed funding and a preclinical snafu derailed the company’s lead development program. But the company stuck with the original idea, eventually navigating through the scientific challenges, and connected with other researchers in their area to refine the focus of the company’s portfolio from broader CNS indications to neurological mechanisms in the respiratory area.
At the BIO meeting in June, company chairman and chief scientific officer Arnold Lippa, Ph.D., told me the long history of RespireRx, known as Cortex until mid-December 2015. It is a tale of persistence, and even audacity, through repeated setbacks and recoveries. The company started up almost four decades ago atop a wave of enthusiasm for ampakines, a class of compounds that enable glutamate receptors to stimulate neural activity in the brain. At the high point of the wave, however, reverses in the field curtailed funding and a preclinical snafu derailed the company’s lead development program. But the company stuck with the original idea, eventually navigating through the scientific challenges, and connected with other researchers in their area to refine the focus of the company’s portfolio from broader CNS indications to neurological mechanisms in the respiratory area.
Planting The Field
The field of neuroscience, or, as the industry prefers, CNS, has been more of a minefield than a productive plantation of new products for some time now. Since Janssen’s haloperidol and Sandoz’s clozapine, and with the notable exception of Prozac and the other serotonin reuptake inhibitors, whole decades have passed without the once-hoped-for advances in CNS drugs. Now, after a long string of faded startups and failed programs in the space, some analysts and investors even counsel new drug developers to avoid CNS entirely.
Against such a background, the relatively few champions in the therapeutic area, like RespireRx, stand out. Building on past failures by others and setbacks of its own, the company has survived mainly by accumulating knowledge and applying a novel idea to a combination of long-term pharmacology and cutting-edge science. And Arnold Lippa has been around to see it all — he was a part of the CNS research universe long before joining the company.
Dr. Lippa came out of the Big Pharma of the 1970s and 1980s, where he ultimately led the molecular neurobiology group at American Cyanamid — then an industry giant that later disappeared in a chain of mergers leading to Pfizer. In 1985, as many of the big players retreated from CNS, Lippa switched to pursuing his scientific interests on the entrepreneurial business side, cofounding and comanaging the former Praxis Pharmaceuticals, followed by others over the next decade.
All the while, he also maintained strong ties with academic research in CNS as an adjunct professor at City College of New York and the NYU School of Medicine and work with the NIH and NSF (National Science Foundation). In the mid-’90s, he founded DOV Pharmaceuticals, another neuroscience development company, which he ran until 2005. He then became a principal and managing member of Aurora Capital LLC, a “small boutique merchant banking house.” Yet, as he now admits, he has always preferred the science side over the business side of the industry.
From the time of Cortex’s inception, Lippa had followed the company closely because of his interest in neuroscience research generally and ampakines specifically. Ampakines are compounds that enhance the actions of glutamate, the primary excitatory neurotransmitter in the brain, at one of its receptors, the AMPA receptor, thus producing electrophysiological and biochemical effects shown to improve cognition and attention deficit disorder. Cortex had focused on a particular subclass of ampakines, based on the work of Dr. Gary Lynch at UC Irvine. Lippa also knew several researchers heading a similar program at Lilly.
“Ampakines were just raging in the scientific community,” he says. “Everyone thought these drugs were going to be memory enhancers, antidepressants, treatments for the cognitive problems in schizophrenia, and so on. Market caps for startups in this space were all over the place. Then the whole movement ran into a brick wall.”
Scientists now know two main types of ampakines exist — high-impact and low-impact. High-impact forms can produce convulsions and neurotoxicity. With candidates in the high-impact category, Lilly shelved its entire ampakine program, which “knocked the wind out” of the space, as Lippa says. Cortex stayed in the game, becoming the leader, because it was developing low-impact ampakines. The low-impact forms did not produce the neurotoxic side effects and still showed promise in improving memory and treating attention deficit disorder.
Then, another lightning bolt struck: In tissue samples from the brains of animals treated with the company’s lead ampakine, CX717, testers found tiny bubbles or vacuoles. “It was like someone had dropped an Alka- Seltzer in water and frozen it,” says Lippa. “That was the death knell for ampakines.”
Rather than surrender and strike the tents, however, Cortex stubbornly but quietly persisted in developing additional low-impact ampakine compounds, digging further into understanding their mechanisms and the reasons for the phenomena that had almost killed the space entirely. As it advanced other compounds, it investigated the “Alka-Seltzer effect” with CX717.
“We now have conclusive data showing that the effect is a postmortem artifact,” Lippa says. “A metabolite of the drug interacts with formaldehyde, the fixative used to preserve the tissue, and the reaction is exothermic; it gives off heat, and it boils the tissue, producing gas bubbles. No vacuoles are observed unless formaldehyde is present.” RespireRx now plans to publish the exculpatory data and hopes to return to the clinic with CX717 in several areas.
Thickening The Plot
 About the same time the company was dealing with the development setbacks — and a brush with bankruptcy — a group led by Dr. John Greer at the University of Alberta identified the presence of neurotransmitter receptors located on brain cells responsible for the central regulation of breathing in a region of the ventral medulla called the pre-Bötzinger complex. The cells contain receptors for opiates as well as for GABA (gamma-aminobutyric acid), the main inhibitory chemical in the nervous system, whose receptors mediate the actions of drugs such as barbiturates, anesthetics, and benzodiazepines.
About the same time the company was dealing with the development setbacks — and a brush with bankruptcy — a group led by Dr. John Greer at the University of Alberta identified the presence of neurotransmitter receptors located on brain cells responsible for the central regulation of breathing in a region of the ventral medulla called the pre-Bötzinger complex. The cells contain receptors for opiates as well as for GABA (gamma-aminobutyric acid), the main inhibitory chemical in the nervous system, whose receptors mediate the actions of drugs such as barbiturates, anesthetics, and benzodiazepines.
“Opiates activate the receptor on a cell that controls respiratory rhythm, impeding the firing of the cell, so breathing slows down, and ultimately the animals die, just like people,” Lippa explains. “The same cells also contain AMPA receptors, and if you give ampakines, you can prevent or reverse the suppression, and you can measure the ampakine effect right at the level of the motor neuron. I believe it is one of the most elegant pieces of translational research ever accomplished.”
The company obtained the patent rights from the research and subsequently, after working with Greer on the breathing-control insight, decided to redirect its entire portfolio into the respiratory-related CNS area. “Breathing is a lot easier to measure than depression,” says Lippa. “It occurred to them — perhaps they should become a breathing company.”
In 2012, the company acquired SteadySleep Rx, founded by Dr. David Carley, a leading respiratory physiologist and sleep researcher at the University of Illinois in Chicago. In an animal model of obstructive sleep apnea, Carley had shown the synthetic cannabinoid dronabinol (THC) could reverse the effects of serotonin on breathing. His company had raised funds and conducted a Phase 2a study, which showed the compound reducing sleep apnea according to the Apnea-Hypopnea Index (AHI). When the two companies merged, financing was scarce, so Lippa formed a new management team with the backing of Aurora Capital.
Lippa’s team blends risk-taking experience with business and industry acumen. James Manuso, who took over as president and CEO when Lippa became the CSO in 2015, has a long history of starting, capitalizing, and running life sciences companies. Lippa’s bank partner, Jeff Margolis, is also an old hand in startup financing and serves as company senior vice president, secretary, and treasurer. The chief financial officer is Robert Weingarten, who has a 30-year history in doing turnarounds. Head of R&D Richard Purcell has extensive experience in the pharmaceutical industry and has run a CRO. “None of us takes a cash salary,” says Lippa. “It’s the only way we could do it. We went through a nonbankruptcy reorganization. Then we got the research program off the ground, and now we’re ready for the next phase — following through with clinical development.”
Wide-Field & Close-Up
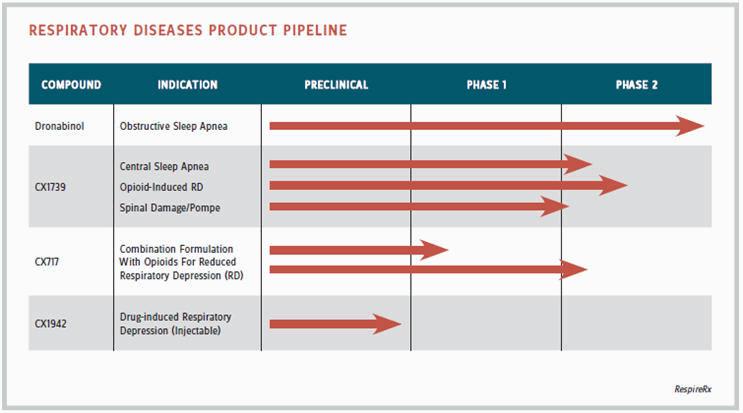
In the broadest possible view, RespireRx may focus on a wide variety of respiratory disorders, according to Lippa. “Sleep apneas, drug-induced apneas, and respiratory depression as a milder form of apnea, central respiratory problems that occur as a result of genetic disorders or injury — our drugs work in many models. For example, we have positive data for the ampakine CX717 in a mutant mouse model of Pompe disease, a muscular dystrophic condition that causes a breathing problem, and in models of spinal injury where respiratory function is also an issue. We are one of the few companies focused on respiratory physiology and pharmacology.”
From a narrower perspective, the company is initially tackling two main areas of related indications — obstructive sleep apnea and central sleep apnea. Its lead product is dronabinol, now nearing the end of a Phase 2 trial in obstructive apnea. Dronabinol is an off-patent drug, approved by the FDA for the treatment of AIDS-induced and chemotherapy-induced cachexia. “Dr. Carley chose dronabinol partly because the track to development is rapid,” says Lippa. “It’s already approved by the FDA, so there’s no long-term animal safety data to do, and we could file an abbreviated new drug application (ANDA).”
Carley recently completed dosing of 120 patients and collected the data in a Phase 2b study of dronabinol versus placebo for six weeks. “It’s potentially pivotal,” Lippa says. “The data is being analyzed, and later this year it will be announced.”
In central sleep apnea, the company has several ampakines in preclinical to Phase 2 development to address the needs of the 11 million chronic opiate patients in the United States. According to Lippa, it is estimated that approximately half of those patients suffer from sleep-related breathing disorders, primarily central sleep apnea. Sleep-disordered breathing is considered a significant risk factor for opiate overdose, leaving about 4.5 million people at risk. In a preliminary clinical trial, CX1739, a lead ampakine, has shown potentially beneficial effects in a small group of patients with central sleep apnea.
Preclinical studies with a number of ampakines, including CX717 and CX1739, have demonstrated their ability to antagonize the respiratory depressant effects of opiates. The most commonly used opiate in these studies is fentanyl, a typical anesthesia in procedures such as colonoscopy and, more recently, appearing in the headlines as the ultimate choice of celebrity and other pain-med users. One of the company’s long-term commercial goals, says Lippa, is to develop a proprietary formulation combining a common prescription opiate with an ampakine — a safer opiate.
Asymmetric Tolerance
As Lippa explains, when more than 30,000 people died in the United States last year from prescription opiate overdose, it was the drugs’ respiratory effect that killed them: “For patients in severe pain, an effective dose of oxycodone is 10 milligrams. You need to take 50 milligrams before you start seeing respiratory depression, but when you start taking opiates chronically, you rapidly develop tolerance. Soon the 10 milligrams doesn’t work, and you go up to 20 milligrams, and after a while, 30, 40, and 50 milligrams. Tolerance develops equally to the euphorigenic effects, the so-called high, but much less tolerance develops to the respiratory depression. The 40-50 milligram dose is bumping up against the level of toxic respiratory depressive effects, and that’s the deadliest problem with the opiate epidemic right now.”
RespireRx has two ampakine candidates for potential opioid-ampakine combinations: CX717, assuming it can be exonerated and taken off the FDA’s negative list, and CX1739. Both will go into a number of yet-to-be-determined studies, all needing additional financing. It is, to say the least, an audacious approach to the opioid dilemma — in keeping with the boldness of the company’s overall thrust into the respiratory area.
“We have three primary goals: use of our products in combination with opiates, in sleep apnea, and in various orphan diseases, starting with spinal injury and Pompe,” says Lippa. “In combination with opiates, there are three potential markets. One is acute and semi-acute use in a hospital or surgical setting. The second is in postsurgical analgesia, as with IV morphine, which is an easy development route because you don’t need long-term animal safety. The third is chronic use in a proprietary combination.”
Is this another comeback story for a waylaid technology, such as monoclonal antibodies or immuno-oncology? Will ampakines enjoy a similar renaissance, reborn in the breathing sphere? It will take further enterprise to find the answer, and the enterprise likely to make that discovery, at the end of its current development trail, now appears to be RespireRx.