Preparing Sanofi For Post-Patent Cliff Product Launches

By Rob Wright, Chief Editor, Life Science Leader
Follow Me On Twitter @RfwrightLSL
 Choosing to come to work for Sanofi in July 2013 couldn’t have been an easy decision for Pascale Witz. After all, she was leaving a job as president and CEO of GE Healthcare’s medical diagnostics business, a company where she had worked for the previous 17 years. Furthermore, Sanofi was facing huge patent cliff hurdles during this time period. Despite all of that, though, Witz saw opportunity in this position as EVP and leader of Sanofi’s newly created global divisions and strategic development organization.
Choosing to come to work for Sanofi in July 2013 couldn’t have been an easy decision for Pascale Witz. After all, she was leaving a job as president and CEO of GE Healthcare’s medical diagnostics business, a company where she had worked for the previous 17 years. Furthermore, Sanofi was facing huge patent cliff hurdles during this time period. Despite all of that, though, Witz saw opportunity in this position as EVP and leader of Sanofi’s newly created global divisions and strategic development organization.
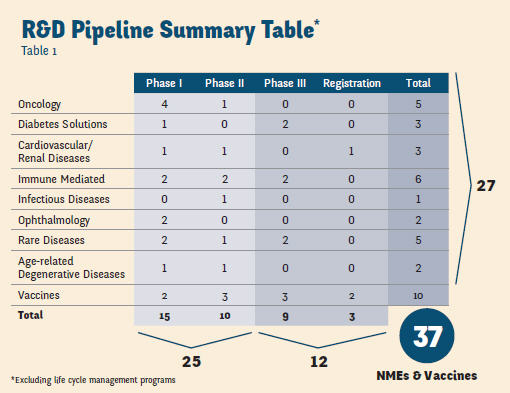
That opportunity was to prepare Sanofi for an unprecedented post-patent cliff drug launch schedule. Part of the rationale for hiring Witz was to bring someone from slightly outside traditional pharma with demonstrated experience in rebuilding organizations, as well as a willingness to do things differently. Here’s why: Since 2009, Sanofi has had the most new drug approvals of any Big Pharma (13), according to Bloomberg analyst report. And though the organization had launched 10 products from 2007 to 2013, Witz knew this pace was likely to soon double. “We could have 18 new product launches in the next five years,” she attests. While you may be thinking this a bit optimistic, consider this: In the past 10 months Sanofi has launched Afrezza (diabetes), Toujeo (diabetes), Lemtrada (MS), and Cerdelga (Gaucher disease) and is readying to launch several other products before year’s end. With 37 compounds in its R&D pipeline (see table on page 35), the challenge for Witz was to build a very different organization than what got Sanofi through its patent cliff period. “During a patent cliff, your focus is on asset maximization, manufacturing cost reduction, and sales efficiency,” she says. “Launching new products today requires you to think more about putting the patient first — building your solutions and offerings around their needs, and seeing how you can make a difference.”

"If you want to improve adherence and change behavior, simplicity of device and diagnostic design is a big driver and should be integrated into your drug development process."
PASCALE WITZ
Executive Vice President (EVP), Global Divisions & Strategic Development, Sanofi
As companies continue to strengthen their biologic pipelines and payers seek to reduce costs while also improving outcomes, Witz believes patients in the near future will have even greater responsibility for managing their treatments. “Most of our new products include devices,” she states, using, as an example, Toujeo, Sanofi’s long-acting human insulin analog that comes in a disposable prefilled pen for management of diabetes. “Early on we focused on the [Toujeo] pen itself, but diabetics will tell you the injection is the easy part. The most difficult part is first determining the dose, which requires getting a blood glucose level.”
Even nondiabetics probably consider the process of conducting a blood glucose test to be fairly common knowledge — pricking a finger with a small lancet device, obtaining a small blood sample, applying a drop of blood to a test strip, inserting the strip into a blood glucose meter, and then getting a blood glucose reading. An experienced diabetic conducts this routine multiple times a day and with probably very little thought to determine the appropriate insulin dose to self-administer. If you can imagine similar processes being applied to other therapeutic areas, you can see where Witz is going. Most biologics require delivery devices. Some even require the use of a companion diagnostic to determine if the patient is an appropriate candidate for a particular therapeutic. With the goal of providing better outcomes, it is not hard to imagine that patients will soon be using multiple drug devices, as well as diagnostics, to deliver and manage their own treatment.
But if better outcomes and lower costs are the goal, more devices and diagnostics aren’t necessarily the solution. Research has shown that despite advances in drug delivery technologies, many patients (84 percent in one study) still do not use these devices properly. “If you want to improve adherence and change behavior, simplicity of device and diagnostic design is a big driver and should be integrated into your drug development process,” says Witz.
Another driver behind Sanofi’s need to change involves the role of payers. “Fifteen years ago, the route of getting an approved drug covered by insurance was much simpler and shorter,” she attests. “Nowadays, the sophistication of the different payers and the complexities of getting patient access to new therapeutics have made insurance companies enormous stakeholders in the drug development process.” No longer can pharma companies assume that if their drug provides clinical benefit it will be covered, and therefore, prescribed. Witz knew that for Sanofi to handle this new pharma business model while planning to launch so many new products, it would need to develop a different organizational structure, a redefined strategy, and a “much more action-oriented mindset.”
To Know Where You Want To Go, First Understand Where You Are
The first thing Witz did, even before arriving at Sanofi, was learn what organizations were already in place and how they were organized. She looked at how people were organized to prepare for the launch of new products and then benchmarked that information against current industry standards and critical functions. For example, she says there are three teams required to launch new products: global brand, marketing, and market access. “At first I was doing two things in parallel — identifying what teams needed to look like and what functions I needed to have access to,” she explains. “Through my initial analysis, I realized that some of these functions were not necessarily well organized for product launch or optimized to leverage all of the technologies available to make a bigger impact.”
Her next step was to determine the critical priorities and timelines. Of course, one of her primary priorities was ensuring Sanofi would be ready to launch its new products coming out of development. That led to a rebuilding of Sanofi’s product launch capabilities (e.g., increased patient engagement, value-driven product development, better integration of combination drug-device development), dubbed product launch excellence, in September 2013. “In October 2013 we started another project designed to define a new market access organization [a center of excellence focused on demonstrating economic value to patients, payers, and regulatory authorities],” she recalls.
Identify The Talent Gaps
To ensure the success of each of the aforementioned initiatives, Witz had to determine where there were talent gaps. She began working with headhunters and HR (see sidebar “Where To Begin Assessing Your Talent” on page 32) to develop desired candidate profiles and draft job descriptions. “Having headhunters review job description drafts can be very helpful to identify the most critical aspects of a job so you don’t end up with a position that looks great on paper, yet will never be able to be sufficiently filled,” she says. This exercise led Witz to conclude that Sanofi needed a new head of global market access, a new head of global marketing, a new head of strategic development, and a number of other positions. For some of the slots that needed to be filled, Witz was deliberate in seeking experience from outside of pharma. For example, when hiring for an integrated care position, she wanted someone with experience in instrumentation, design software, and devices. “I felt neither Sanofi nor pharma had people with the level of experience I wanted in these areas,” she says. “Experience, as well as having the right mix of people, matters.” Other attributes of importance for Witz include leadership and the ability to come up with ideas and recommend a path and defend it. “I want people who are self-confident and can lead by example so they can shape the company by exerting influence beyond their direct team,” she states.
For a newly created position, chief patient officer, Witz wanted someone who was very patient-centric and not colored by the way traditional pharma operates. “Filling this position was actually very difficult because I wanted a mindset more than a profile,” she explains. “For that specific hire, I had to actually argue with the headhunter who initially wanted me to target somebody with pharma R&D experience.” Though Witz conducted a few interviews with folks having this background, she found many commenting how they would be good at telling R&D how to work. “That’s the last thing I wanted,” she says. “I wanted someone who was working with R&D, with my commercial team, and with my division, not somebody who was telling others what to do.” Sticking to her original plan, Witz eventually landed Anne Beal, M.D., MPH, a pediatrician and public health specialist from the Patient Centered Outcomes Research Institute (PCORI) — the United States’ largest institute focused on patient-centered outcomes research. The move made Sanofi the first top 10 Big Pharma to create and fill a chief patient officer position. “I strongly believe that innovation comes from creating a convergence of different fields,” she attests. “If you want to better understand the patient’s perspective, bring in someone with significant patient experience. If you want to better understand how to develop combination drug devices, acquire people with device experience.”
Witz shares that, though Sanofi did have a lot of very good people internally, some were not at the right level of competency. “It was more a question of training,” she says. Her advice is not to compromise and place someone in a role beyond their skill level, but instead do what she did — create a mentor system to build your internal capabilities.
Another tip, don’t use the same headhunter to fill every position. “When you are in a position to build the team, talk to different headhunters to tap into a variety of experiences and perspectives,” she says. “A lot of the roles to be filled were going to be a part of my leadership team. For the headhunter to really understand what I was looking for required me to be quite engaged.” While Witz believes that building the right team requires having an HR partner you trust, recruiting top talent is not something to be completely delegated. “There are many areas where I don’t have the expertise, and I’m fine with that,” she states. “My role as a leader is to be able to lead a team with complementary knowledge and skills. While this requires being able to get along with and trust the people you hire, because many positions were being filled by nontraditional pharma people, I could not just hand it over to HR and let them handle it. Even when I look back, I don’t think this is something that I could really delegate as I will be relying on these people for their expertise.”
Since spearheading the integration of Sanofi’s burgeoning product pipeline with its product launch capabilities and its patient-centric initiatives, Witz has been very busy. In just over two years, she has successfully filled a series of key positions such as chief patient officer, head of strategic development, head of marketing, head of market access, business leaders for specialty care division and biologics division, and repositioned internal staff to gain better functional and reporting alignment. Despite all that, several initiatives remain works in progress. In order for Sanofi to achieve its goal of reflecting the future of biopharma (i.e., patient-centricity), it requires a continued focus on shifting its culture toward a model that integrates patient care with disease-specific drug/device development.
During the writing of this article, Sanofi announced a new global business unit structure. Consisting of five business units (general medicines & emerging markets, specialty care, diabetes & cardiovascular, Sanofi Pasteur, and Merial), the new structure will go into effect in January 2016. Peter Guenter, former EVP, global commercial operations, will head up the general medicines & emerging markets global business unit. David Meeker, former EVP and CEO of Genzyme, will lead the specialty care global business unit. The diabetes & cardiovascular global business unit will be led by Pascale Witz. Sanofi Pasteur and Merial will be led by their current respective leaders, Olivier Charmeil and Carsten Hellmann. The composition of the executive committee remains unchanged.
Where To Begin Assessing Your Talent
For Pascale Witz, EVP and leader of Sanofi’s global divisions and strategic development organization, the first place to begin assessing your people begins with human resources. “Whenever I start a new role, one of the first assessments is with the head of HR,” she says. “You need someone in this position that is a strategic thinker when it comes to staffing so you can have greater focus on the business imperatives.” While there are a number of assessment tools that can determine one’s ability to think strategically, Witz pays close attention to how someone engages with her. “A strategic thinker is not afraid to challenge you if they think you are going in the wrong direction,” she attests. “It is a bit of a gut feel, and often based on the questions they’re asking me.” A taskoriented HR person asks what type of people you want. Someone more strategic helps you figure out the type of people you need. Witz says to be wary of an HR individual who is too much into praising how many people he or she has hired in the past. “I’m not looking for metrics in terms of number of people,” she explains. “I’m looking for experience in difficult roles. I want people who will tell me if the organization I am developing will work or not and help me to make sure the plan looks, and is, simple.” One of Witz’s first tasks was bringing in someone from outside of Sanofi as her new head of HR.
Another assessment conducted by Witz shortly after her arrival at Sanofi involved the people focused on product launch. Beginning with conversations among her team and then division heads, Witz soon learned that, although Sanofi did have some folks in house, the reality was that, given pipeline projections, they didn’t have nearly enough people for this task. According to Witz, the initial assessment revealed there was no existing team with recent product launch experience. Witz pulled together the handful of people who did have previous launch experience so she could ascertain where Sanofi had gaps relative to its developing pipeline. “This is how we scoped the organization and concluded the jobs that would need to be created. Then we looked at who was qualified, interested, and available internally who could fill those roles prior to looking outside,” she explains.
Getting Organized Required Getting Help
The opportunity to create an integrated care initiative was one of the things that attracted Pascale Witz to join Sanofi. The EVP and leader of Sanofi’s global divisions and strategic development organization believed that such an initiative required the creation of five centers of excellence: integrated patient care, patient-centricity, marketing, market access, and global strategic development. When embarking on creating the market access center of excellence, which focuses on demonstrating economic value to patients, payers, and regulatory authorities for newly developed therapeutics, one thing became clear — Sanofi had a lot of different subfunctions of market access spread out across the company. Given the size of the project, Witz also quickly realized that she needed the help of someone she could trust. “I decided to hire an expert consultant who had worked in a role linked to market access whom I had worked with previously,” she says. “But I also built a project team and named an internal project leader to work hand-in-hand with the consultant. I’m not a big fan of just having a completely external assessment without pairing them with somebody internal who can adjust and course correct.” Witz admits to having a strong opinion on this point. “Consultants deliver based on how much you guide them. While sometimes it is appropriate to have an assessment done by somebody who is completely external, when it includes either business strategy or organizational structure, you need someone on the inside who can help open doors and guide the consultant — as well as catch any misperceptions that might begin to take shape internally.”
Working together, the two began conducting a full assessment of Sanofi’s market access capabilities, a process that took two months. One of their conclusions was that, in order to accelerate Sanofi’s market access capabilities and make sure people were working together, they had to be unified under one global function and reporting structure. “We rewrote the health economic outcome research, the pricing, the different divisions, and the market access keys and then created a market access academy to train people so we could ramp up our market access competencies. We then put an operations team in charge of managing the training academy,” says Witz.
