Use Of TMF Solutions In Clinical Development Studies
By Industry Standard Research
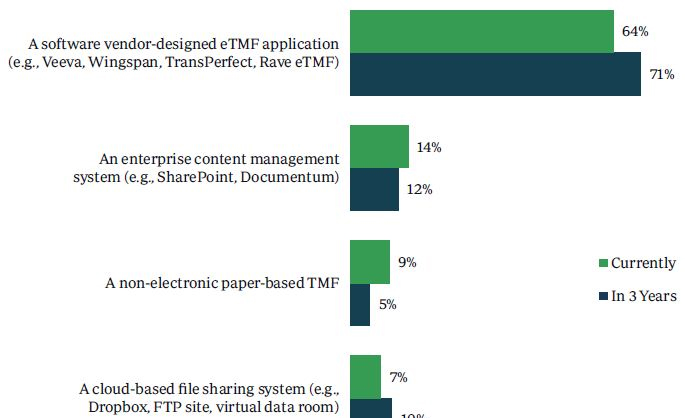
Nearly two-thirds of clinical development studies (64%) are currently using a software vendor-designed eTMF application such as Veeva Vault, Wingspan Technology, TransPerfect Trial Interactive, and Medidata Rave eTMF. A slight increase (71%) is expected three years from now. The next most commonly used solution, an enterprise content management system such as SharePoint and Documentum, has considerably less use at only 14%.
“Currently, of the clinical development studies you are familiar with, what proportion of them use the following TMF solutions as a document management solution? Your answers should sum to 100%.” (n=110)
“Thinking three years from now, what proportion of the clinical development studies at your company use the following TMF solutions as a document management solution? Your answers should sum to 100%.” (n=110)