Benefits Of Clinical Development Outsourcing Models
By Industry Standard Research
ISR’s Clinical Development Outsourcing Models (5th Edition) report examines three outsourcing models commonly used in the clinical trial space. Respondents provided feedback on the benefits of each outsourcing strategy currently used at their company.
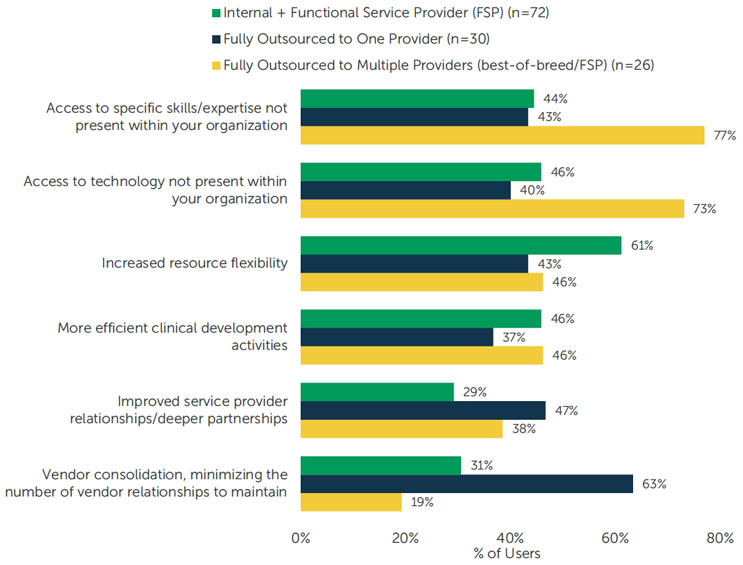
Internal + Functional Service Provider (FSP): A sponsor executes some clinical development activities for a study internally but outsources all or most of one or multiple functions (e.g., data management, biostatistics, pharmacovigilance, etc.).
- Top benefit: Increased resource flexibility (61%)
Fully Outsourced to One Provider: A sponsor outsources all services for a study to a single CRO. The CRO may or may not subcontract to other providers.
- Top benefit: Vendor consolidation, minimizing the number of vendor relationships to maintain (63%)
Fully Outsourced to Multiple Providers (best-of-breed/FSP): A sponsor fully outsources the execution of a study but contracts directly with various “best-of-breed” providers to mix and match services for a custom approach to completing a clinical development project.
- Top benefits: Access to specific skills/expertise not present within your organization (77%) and Access to technology not present within your organization (73%)
“Please select the benefits you experience from using the [Internal + Functional Service Provider (FSP)/Fully Outsourced to One Provider/Fully Outsourced to Multiple Providers] model for outsourcing. Select all that apply.” (n=varies)