Bioprocessing Sees Continued Improvements In Batch Failure Reductions In 2022
By Joel Ranck, BioPlan Associates

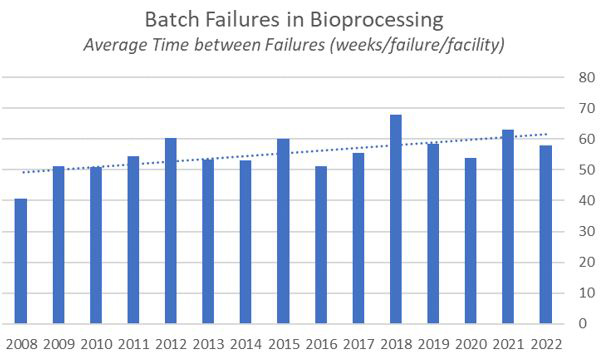
Bioprocessing is generally doing better than ever in terms of minimizing batch failures. Over the past 15 years we have been measuring failures in our annual report, and we have seen an overall improvement of around 11 weeks on average between batch failures. This continues a gentle upward trend in quality manufacturing.
Although this year the average interval between batch failures of 58 weeks is down from 63 weeks in 2021, the industry is clearly maturing. In BioPlan Associates’ 19th Annual Report and Survey on Biopharmaceutical Manufacturing Production and Capacity, which surveys more than 140 biopharmaceutical companies and 130 industry suppliers and vendors, we found that operator error was the leading cause of batch failures (accounting for 3.8% on the commercial scale and 3.8% on the clinical scale) in 2022.
Previously, batch failures were blamed on equipment failures or contamination. When the equipment was the leading cause of batch failure, fixing the equipment or implementing new technology was an obvious solution. Fixing operator errors will be more difficult, and the time it will take to recruit, train, and retain the right operators may be such that operator error could be the leading cause of batch failures for years to come.
Fig. 1: Average Batch Failure Rates in Bioprocessing, Weeks per Failure 2008–2022
Equipment Failure Still Looms Large
Operator error has been a top-three cause of batch failures for years, but contamination and equipment failure have captured the attention of the quality assurance professionals, and rightly so. This year, at the commercial scale, “Equipment Failure” is the second most reported cause of batch failure at 3.3%, and “Contamination” is the third most reported cause (Fig. 2).
Fig. 2: Batch Loss Primary Causes by Scale of Manufacture, 2022
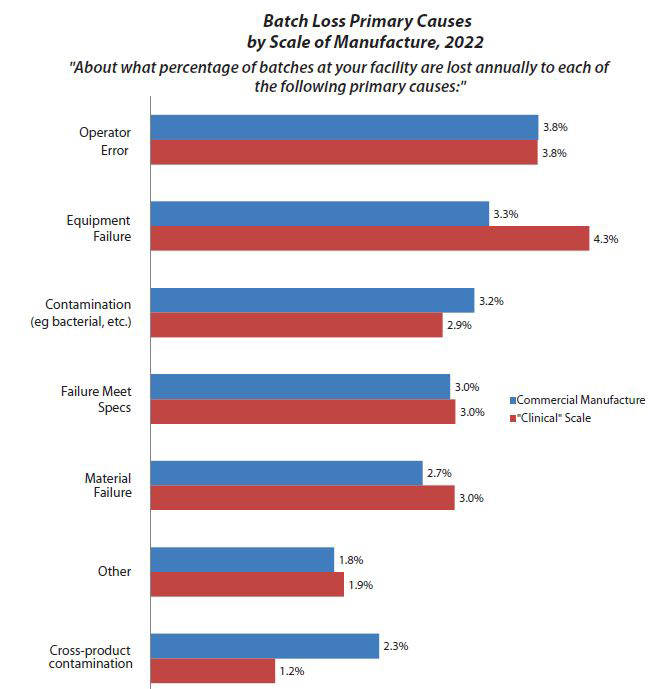
Obviously, any failures at a commercial scale are very serious and costly. Besides often having to redo the bioprocessing and potentially disrupt product marketing and sales (which can be disastrous), costly and time-consuming forensic studies of reasons for failures may be required to satisfy regulators. In general, many of the failures can likely be traced to a need for better training or other improvements in working conditions, such as allowing more time for staff to perform processes and more oversight of operators. In terms of contamination-related failure rates, it is expected that they would be higher for clinical manufacturing. Similarly, it is rather reasonable to expect more failures attributed to equipment at earlier clinical vs. later-stage commercial manufacturing.
In this year’s survey, we see the biggest differences between commercial manufacture and clinical scale with equipment failure and cross-product contamination. Is this a big deal? Yes and no. It’s important because once you identify the problem you can focus on fixing it. It’s not such a big deal because this is nothing new to any of us, since operator error has ranked in the top three causes of batch failures for the past decade or more.
Increased Focus On Human Resources
But what makes operator error an important factor to focus on now lies both in the fact that batch failures are becoming less common over time due to interventions and changes in the industry and that hiring expert operators has become so difficult in recent years. In 2022 there’s the opportunity to reduce operator error by hiring and training more experts.
Now, here’s the rub. The 19th Annual Survey indicates that hiring experts is becoming more and more difficult. The number of Ph.D.-level experts available has shrunk and competition for them has increased. The strategy of many companies to hire in advance of expected expansions has further shrunk the pool of candidates to make a difference in batch failures due to operator failure. However, for those who have increased their bench strength in advance of ramping up operations, this is the ideal time to begin training those employees and to use them to train other staff on good manufacturing practices to reduce batch failures.
Fig. 3: Estimated New Staff Hires in Biopharmaceutical Production Facilities in Five Years (2027)
Roughly 15 years ago, BioPlan Associates wrote in the 5th Annual Survey that the average interval between batch errors was about 40 weeks. Today that interval is 58 weeks. Even 14 years ago, the recommendation was to increase training to decrease operator error. Still, the low-hanging fruit was to focus on the hardware. In recent years we’ve seen more single-use and disposable materials that have reduced the incidence of contamination. Continuous bioprocessing is on the rise, which also contributes. Further improvements in warehousing, freezing, and thawing also have minimized breakage and losses in transportation. All of this has gone to improving the interval between batch failures.
The next frontier in reducing batch failures must focus on human resources, just as past innovations focused on hardware. Low unemployment, the Great Resignation, and the lingering symptoms of the pandemic on the employment market have contributed to the ongoing and incrementally worsening bottleneck in finding, training, and keeping the right staff with the right expertise. This ranges from training someone capable of managing a unit operation to hiring a manager capable of effectively operating a GMP facility in a highly regulated environment and to hiring a specialist in bioprocess design or optimization.
Long-term Investment Required
Change will not be instant. Once graduates are hired, they tend to spend six to 12 months in training to gain the needed hands-on knowledge and expertise. Often, this training involves simply understanding the importance of doing things a certain way or learning the relevant standard operating procedures. Once staff reach that point, they have become rather valuable, and the potential risk of their being hired away increases. Thus, we see a close connection between the hiring, training, and retention of employees involved in bioprocessing; and there is a healthy amount of generic knowledge and technology transfer among facilities over time.
The importance of suitably and fully trained staff is shown again by the data this year. The growth and importance of commercial product manufacturing, with more products entering the market, including biosimilars and emerging technologies like cell and gene therapies and pandemic vaccines and therapeutics, means that more process development and bioprocessing are being done relative to basic research. And process development and other bioprocessing fields will be competing for trained, skilled workers with manufacturing and regulatory expertise.
Conclusion
Overall, the market for bioprocessing staff expertise will remain moderately tight and will likely
slowly worsen, as many of the most experienced baby boomer staffers retire and pandemic vaccines and their companion therapeutics bioprocessing add to demand. One of the main ways needed skilled employees are obtained is by “poaching” from one company to another, with every company affected. Therefore, retaining skilled employees should become as much a focus as training them.
One solution might be refocusing efforts from hiring Ph.D.s to partnering with undergraduate institutions and community colleges to develop a workforce more familiar with commercial-scale manufacturing. This might also require an increase in management to train and oversee this new workforce, but it should reduce the turnover inherent with highly trained Ph.D.s being poached or simply getting bored and returning to academia.
What does this mean regarding batch failures? We can expect to see the average gap between batch failures grow from 58 weeks to 70 weeks over the next decade, but those gains will be harder earned. There continues to be room for growth of continuous bioprocessing and single-use systems that will decrease the instance of contaminations that result in batch failures. However, without a comprehensive human resources strategy, operator error as the leading cause of batch failures might be the number one cause of batch failures for years to come.
References
- 19th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production, BioPlan Associates, Inc. 528 pages, April 2022. www.bioplanassociates.com
About The Author:
 Joel Ranck is a market strategist with extensive experience in science, medicine, public health, healthcare, research, and higher education. His expertise includes primary and secondary research and market analysis of biopharma segments. His work has spanned the globe from Central Europe to South America and the U.S. He can be reached at jranck@bioplanassociates.com, +1 301-921-5979, www.bioplanassociates.com.
Joel Ranck is a market strategist with extensive experience in science, medicine, public health, healthcare, research, and higher education. His expertise includes primary and secondary research and market analysis of biopharma segments. His work has spanned the globe from Central Europe to South America and the U.S. He can be reached at jranck@bioplanassociates.com, +1 301-921-5979, www.bioplanassociates.com.
