Biosimilar Litigation Review: Ongoing BPCIA District Court Cases To Watch
By Philip Chen, Felix Eyzaguirre, Tasha Francis, and Jenny Shmuel, Fish & Richardson P.C.

In many ways, last year was a notable one for biosimilars in the U.S. During the 2019 calendar year, the FDA approved the 26th biosimilar product, and the 12th biosimilar product was launched in the U.S. market (with more expected to launch in the coming weeks). These developments were accompanied by a flurry of activity at the FDA, new proposed and enacted legislation, and many new developments in court and in post-grant proceedings. This four-part article series will focus on 2019 legal developments related to the biosimilar sector — specifically, litigation under the Biosimilars Price Competition and Innovation Act (BPCIA), litigation related to alleged anticompetitive behavior on the part of reference product sponsors, and biologic-related post-grant challenges.
BPCIA Litigation
BPCIA litigants and district courts grappled with new issues in 2019. The Federal Circuit has also been busy, with numerous BPCIA issues pending and a handful of new decisions issued last year. In Part 1 of this article series, we briefly summarize overall statistics regarding BPCIA district court litigation and review ongoing BPCIA district court cases. Subsequent installments in the series will discuss BPCIA district court cases that settled in 2019 and pending and newly decided BPCIA appeals.
Since the BPCIA’s enactment in 2010, over 40 BPCIA cases have been filed in district courts. (See Figure 1.) Amgen (with 15 cases) and Genentech (with 13 cases) are the most active plaintiffs and together account for the plaintiff side in more than half of all BPCIA litigation to date. Amgen is also the most common BPCIA defendant (with eight cases), while Celltrion (defendant in seven cases) and Sandoz (defendant in six cases) are not far behind. BPCIA litigation has involved biosimilars of nine different reference products: Remicade, Neulasta, Neupogen, Avastin, Herceptin, Rituxan, Humira, Enbrel, and Epogen. Unsurprisingly, these mirror the nine reference products for which there are FDA-approved biosimilars. The District of Delaware, the District of New Jersey, and the Northern District of California have emerged as the most common venues for BPCIA litigation.
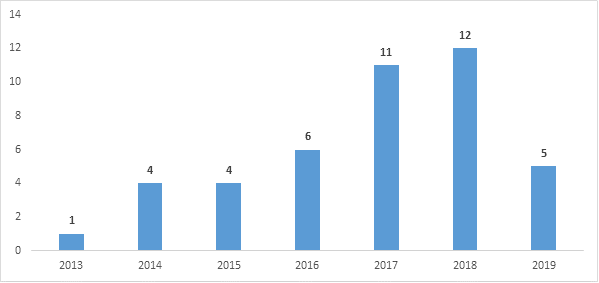
Figure 1: BPCIA cases filed by year since BPCIA enactment
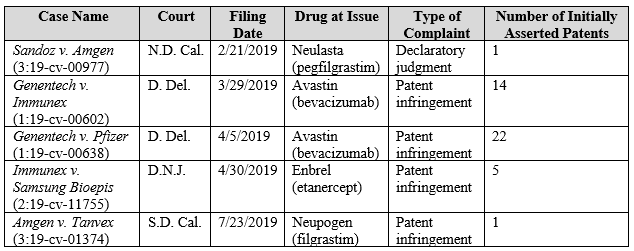
2019 saw a decrease in new BPCIA district court filings in comparison to the last three years (see Figure 1). Further, several of the 2019 cases were follow-on cases to add new patents, as opposed to new disputes involving new parties and/or biosimilars. Of the five new BPCIA district court cases filed in 2019, only Genentech v. Immunex and Immunex v. Samsung Bioepis are still ongoing; the remainder have been dismissed. The newly filed BPCIA cases are summarized in Table 1 and are discussed individually in the section addressing ongoing litigation below and in the review of litigation resolved in 2019 in Part 2.
Table 1: BPCIA Cases Filed in 2019
Ongoing BPCIA District Court Litigation
As discussed above, the BPCIA cases filed in 2019 that are currently ongoing include:
- Genentech v. Immunex (19-cv-00602 D. Del.); Avastin/Mvasi
- Immunex v. Samsung Bioepis (19-cv-11755 D.N.J.); Enbrel/Eticovo
In addition, several cases filed prior to 2019 remain ongoing:
- Genentech v. Amgen (17-cv-01407 D. Del.); Avastin/Mvasi
- Genentech v. Amgen (18-cv-00924 D. Del.); Herceptin/Kanjinti
- Amgen v. Hospira (18-cv-01064 D. Del.); Neupogen/Nivestym
- Amgen v. Coherus (17-cv-00546 D. Del.); Neulasta/Udenyca
We discuss each ongoing BPCIA district court case briefly below.
Genentech v. Immunex (19-cv-00602 D. Del.)
This case concerns Mvasi, Amgen’s biosimilar to Genentech’s Avastin (bevacizumab), and relates to two prior cases between the parties consolidated into Case No. 17-1407 (D. Del.).
Genentech filed this new suit in response to a supplement to Amgen’s biologics license application (BLA) for Mvasi filed in August 2018 and produced during litigation in the previous cases. Genentech contended that a supplement to the BLA was an “application” within the meaning of § 262(l)(2), thus triggering the contention-exchange process and other aspects of the BPCIA “patent dance” (Dkt. 24 at 5). In a heavily redacted complaint, Genentech asserted 14 patents, including 12 that it had asserted previously in Case No. 17-1407, and two new patents directed to manufacturing processes, U.S. Patent Nos. 9,493,744 and 9,714,293 (Dkt. 12).
On May 13, 2019, Amgen moved to dismiss the complaint for failure to state a claim. Amgen argued that plaintiffs “missed the deadline to request leave to add these [two new] patents to the 1407 Case (because they did not have good cause to do so) and now hope to sidestep the Court’s good cause requirement” (Dkt. 20 at 1). Among other allegations, Amgen alleged that Genentech was engaging in “claim-splitting” based on the “groundless premise that a new round of the BPCIA exchange process automatically begins, and a follow-on complaint may be filed, whenever a biosimilar applicant submits a supplemental BLA for approval of an [redacted] for an already-approved product” (Id. at 2). Genentech opposed dismissal (Dkt. 24). No decision on this motion is available on the public docket.
In July 2019, when Amgen announced it was preparing to immediately launch Mvasi, Genentech filed two emergency motions seeking to enforce the BPCIA’s 180-day notice of commercial marketing requirement in connection with the new supplements and a temporary restraining order (TRO) to prevent potential marketing of Mvasi (Dkt. 29; Dkt. 31). Although Amgen had provided notice of commercial marketing back in October 2017, Genentech argued that new notice was required because “Mvasi is a new product … accompanied by a new label, and the subject of several applications, FDA reviews, and FDA approvals” (Dkt. 50 at 10). The court denied both motions (Dkt. 47), which Genentech immediately appealed on July 19, 2019 (Dkt. 43). The appeal, CAFC 19-2155, will be discussed in Part 3 of this article series. That same day, the district court also denied Genentech’s motion for an injunction pending appeal. Amgen launched Mvasi in the United States in July 2019.
Immunex v. Samsung Bioepis (19-cv-11755 D.N.J.)
This case involves the reference product Enbrel (etanercept) and Samsung Bioepis’ biosimilar, Eticovo. On April 30, 2019 — only five days after FDA approved Eticovo — Immunex asserted five patents against Samsung Bioepis, three of which were owned by Immunex and two that Immunex had exclusively licensed from Roche (Dkt. 1 at 3). Samsung Bioepis answered the complaint on August 5, 2019 (Dkt. 70).
Early in the litigation, on May 29, 2019, Sandoz moved to intervene in this case with a heavily redacted brief in support of the motion (Dkt. 37). In a separate lawsuit with Immunex, Immunex v. Sandoz (16-cv-01118 D.N.J.), Sandoz had challenged validity of the Roche-owned patents, U.S. Patent Nos. 8,063,182 and 8,163,522 (Id. at 10). In its brief, Sandoz claimed it had a “substantial and central interest in the property that is the subject of the litigation” that may be impaired, and that its interests were not adequately represented by the parties (Id. at 6, 8-9). The court has not ruled on Sandoz’s motion.
On December 10, 2019, a scheduling order issued, setting the close of fact discovery for December 21, 2020, and the close of expert discovery for April 13, 2021 (Dkt. 101 at 3-4). On January 6, 2020, Judge Cecchi entered a stipulation and order, which is currently under seal.
Genentech v. Amgen (17-cv-01407, 17-cv-01471 D. Del)
This litigation involves Amgen’s biosimilar version of Genentech’s reference product Avastin (bevacizumab). This case saw significant activity in 2019 as the parties engaged in fact discovery.
One notable dispute arose over waiver of attorney-client privilege in both this case and Civil Action No. 18-cv-00924 related to Herceptin. Genentech had asserted claims of willful infringement, to which Amgen responded with an advice-of-counsel defense. In an order dated June 20, 2019, the court found that “Amgen’s production of its opinion letters … has effected a subject matter waiver of Amgen’s attorney-client privilege concerning infringement and validity of those patents,” and the waiver “extends to communications pre-dating the opinion letters and extends to Amgen’s in-house counsel” but not “outside trial counsel” (Dkt. 407 at 1). Amgen filed a motion for reargument (Dkt. 423), which the court denied (Dkt. 488).
The court also granted-in-part a motion by Amgen to compel Genentech to produce “licensing and/or settlement agreements” with other biosimilar developers, including Pfizer and Celltrion (Dkt. 387). The court required Genentech to produce “any other terms … that have any relevance to the value placed upon any of the patents implicated therein, including but not limited to royalties, lump sum payments, or any other consideration identified in the agreements” (Id.).
A jury trial is set for November 30, 2020.
Genentech v. Amgen (18-cv-00924 D. Del.) (TRO/PI Appeal = CAFC 19-2156)
This case, filed in June 2018, relates to Genentech’s Herceptin (trastuzumab) and Amgen’s biosimilar, ABP 980, marketed under the tradename Kanjinti.
On July 10, 2019, Genentech filed a motion for a TRO and preliminary injunction to prevent Amgen from commercially launching Kanjinti until the court rendered a decision on the merits following a full trial (Dkt. 308). The court denied the motion, finding that Genentech waited too long to file: “fourteen months after receiving the Notice of Commercial Marketing, three months after receiving a fairly specific launch date, and almost one month after Amgen had FDA approval to launch Kanjinti” (Dkt. 315 at 5). The court determined that Genentech’s undue delay was sufficient to deny its motion and that Genentech would not suffer irreparable harm due to its previous licensing of certain asserted patents (Id. at 6). Genentech appealed on July 19, 2019 (CAFC 19-2156, which will be discussed in Part 3). Genentech launched Kanjinti in July 2019.
A jury trial is scheduled to start on April 20, 2020.
Amgen v. Hospira (18-cv-01064 D. Del.)
This case involves Nivestym, Hospira and Pfizer’s biosimilar of Amgen’s Neupogen (filgrastim). On July 18, 2018, Amgen asserted one patent in its initial complaint: U.S. Patent No. 9,643,997, directed to protein purification (Dkt. 1 at 3).
The parties are currently engaged in expert discovery. A jury trial is set for June 15, 2020 (Dkt. 26).
Amgen v. Coherus (17-cv-00546 D. Del.)
This case involves Amgen’s Neulasta (pegfilgrastim) and Coherus’ biosimilar Udenyca / CHS-1701. The District of Delaware granted Coherus’ motion to dismiss, finding that Amgen was barred by both prosecution history estoppel and the disclosure-dedication doctrine from asserting infringement under the doctrine of equivalents. The Federal Circuit affirmed on July 29, 2019, as will be discussed in Part 3.
In October 2019, Coherus filed a motion seeking to recover its attorneys’ fees (Dkt. 92). Coherus argued that this case was exceptional for a number of reasons, including the weakness of Amgen’s infringement case and Amgen’s insistence on litigating all the way through appeal (Dkt. 92, at 6–11; Dkt. 97). Amgen opposed (Dkt. 95). The motion is still pending before the court.
Part 2 of this four-part article series provides a review of BPCIA district court cases that settled in 2019.
 About The Authors:
About The Authors:
Philip K. Chen is an associate at Fish & Richardson, where he works on various patent litigation matters including pharmaceutical and biosimilar litigation. He can be reached at pchen@fr.com.
 Felix A. Eyzaguirre is an associate at Fish & Richardson, where he focuses his practice on patent litigation, including pharmaceutical litigation. He can be reached at eyzaguirre@fr.com.
Felix A. Eyzaguirre is an associate at Fish & Richardson, where he focuses his practice on patent litigation, including pharmaceutical litigation. He can be reached at eyzaguirre@fr.com.
 Tasha M. Francis, Ph.D., is an associate at Fish & Richardson, where she focuses her practice on patent litigation. She is a well-known speaker and writer on topics related to the Biologics Price Competition and Innovation Act and life science post-grant proceedings. Her clients range from emerging companies in the biotechnology and biopharma industries to established international pharmaceutical companies. She has extensive experience in the areas of small molecule and biologic pharmaceuticals (including antibody technologies), drug formulation and drug delivery technologies, diagnostics, protein biochemistry, and chemicals. She can be reached at tfrancis@fr.com.
Tasha M. Francis, Ph.D., is an associate at Fish & Richardson, where she focuses her practice on patent litigation. She is a well-known speaker and writer on topics related to the Biologics Price Competition and Innovation Act and life science post-grant proceedings. Her clients range from emerging companies in the biotechnology and biopharma industries to established international pharmaceutical companies. She has extensive experience in the areas of small molecule and biologic pharmaceuticals (including antibody technologies), drug formulation and drug delivery technologies, diagnostics, protein biochemistry, and chemicals. She can be reached at tfrancis@fr.com.
 Jenny Shmuel, Ph.D., is a principal at Fish & Richardson, where she has helped build the firm’s biologics and biosimilar litigation practice. She also represents pharmaceutical clients in competitor litigation and Hatch-Waxman litigation. Dr. Shmuel has extensive experience in all phases of litigation, and, over the last several years, has helped successfully defend a large pharmaceutical franchise and secure a significant damages award for a medical device manufacturer. She can be reached at shmuel@fr.com.
Jenny Shmuel, Ph.D., is a principal at Fish & Richardson, where she has helped build the firm’s biologics and biosimilar litigation practice. She also represents pharmaceutical clients in competitor litigation and Hatch-Waxman litigation. Dr. Shmuel has extensive experience in all phases of litigation, and, over the last several years, has helped successfully defend a large pharmaceutical franchise and secure a significant damages award for a medical device manufacturer. She can be reached at shmuel@fr.com.
The opinions expressed are those of the authors on the date noted above and do not necessarily reflect the views of Fish & Richardson P.C., any other of its lawyers, its clients, or any of its or their respective affiliates. This post is for general information purposes only and is not intended to be and should not be taken as legal advice. No attorney-client relationship is formed.
