Implementing Continued Process Verification With Bio4C ProcessPad™ Software
Source: MilliporeSigma
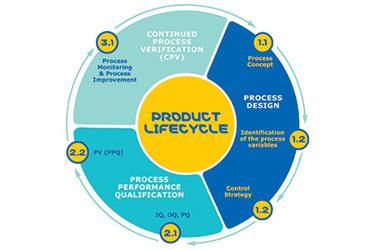
Continued Process Verification (CPV) is the lengthiest and most data heavy phase of process validation. It requires continuous monitoring of processes against defined limits (specification, action and statistical control limits) throughout the commercial life of a product. In this eBook, we describe how Bio4C ProcessPad™ software automates many steps involved in continued process verification and offers a one-click solution for statistical trending of data (control charts, correlation charts, box plots, etc.), campaign reports, and annual product quality review (APQR).
access the E-Book!
Log In
Get unlimited access to:
Trend and Thought Leadership Articles

Case Studies & White Papers

Extensive Product Database

Members-Only Premium Content

Welcome Back! Please Log In to Continue.
X
Enter your credentials below to log in. Not yet a member of Life Science Leader? Subscribe today.
Subscribe to Life Science Leader
X
Subscribe to Life Science Leader
MilliporeSigma
This website uses cookies to ensure you get the best experience on our website. Learn more
