Laying The Foundation For One-Time Therapeutic Reimbursement
By Gail Dutton, Contributing Writer
Follow Me On Twitter @GailLdutton
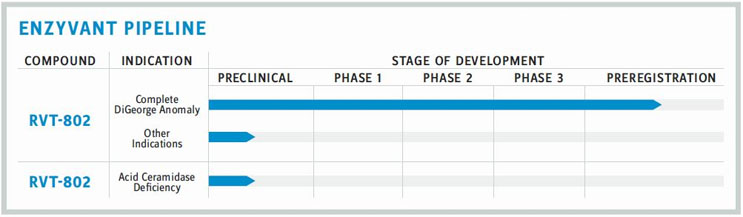
Today’s reimbursement systems aren’t set up to deal with one-time therapies. One company that is well aware of this shortcoming is Enzyvant. With a focus on developing treatments for rare and ultrarare diseases, Enzyvant is currently nearing final submission of a BLA (biologic license application) for RVT-802, a tissue-based regenerative therapy that has the potential to cure complete DiGeorge Anomaly (cDGA) with one administration. The company expects the BLA to be reviewed and RVT-802 to be potentially approved in 2019, so solving the reimbursement issue is urgent for both the company and for patients.
"CDGA is extremely rare,” points out Alvin Shih, M.D., Enzyvant’s first CEO. “It affects 10 to 20 infants in the U.S. [each year] and results in immunodeficiency. Most patients will die by age two or three if they are not treated.” For a new reimbursement strategy, “RVT-802 is an ideal test case because the outcome is clear and measurable.”
To lead and help the company transition from R&D to hopefully its first approved therapy, Enzyvant hired industry vet Rachelle Jacques, most recently at Alexion, as CEO in February. Jacques brings significant experience in commercial leadership and launching rare-disease treatments, which will be pivotal for the company moving forward. As it prepares for potential approval, the company is working with payers and regulators to shape a coverage and reimbursement model that works for regenerative therapies and is hoping to launch a pilot program. Payers have an interest in figuring out the right model as well, given the increasing number of one-time therapies being approved or in late-stage development.
“This is an exciting time for the company,” says Jacques. “If we get this right, we’ll not only benefit the cDGA community by providing access to an approved therapy, but we will help other rare disease communities and companies by advancing a commercial model that might work for them as well.”
Reimbursement for one-time therapies is unlike that for chronic conditions, because the financial risk in the current system is borne by a single payer, all at once, whereas chronic conditions may be reimbursed by many payers over the patient’s lifetime. Launching a pilot requires payers who are willing to share such risk with drug developers as the price of advancing more effective therapies. Doing so would help offset or eliminate the high costs they now pay for ongoing care, support services, surgeries, hospitalizations, and related expenses of patients without access to treatments.
![]()
"We are now poised, just a couple years in, to potentially see our first approval later this year."
RACHELLE JACQUES
CEO, Enzyvant
A CUSTOMIZED FDA FRAMEWORK HAD TO BE DEVELOPED
RVT-802 is alone in a new therapeutic category, which only adds to the complexity of getting this one-time treatment to market. (The FDA had no applicable approval process for it.) Enzyvant worked closely with Duke University (the therapy’s codeveloper) and the FDA to identify the assays and tests that could be used to demonstrate safety, efficacy, and quality. In addition, the company developed a custom algorithm and applied advanced analytics to mine historical data that would help to better predict and assure present and future product quality for this complex therapeutic. This approach of involving academic and industry partners while closely consulting with regulators paid off; RVT- 802 received an unprecedented combination of Breakthrough Therapy, Regenerative Medicine Advanced Therapy, Rare Pediatric Disease, and Orphan-Drug designations from the FDA.
THE UNIQUE NATURE OF RARE DISEASE R&D
As the company juggles its BLA submission for RVT- 802 and other development activities, Jacques recognizes the unusual challenges faced by companies pursuing treatments for rare diseases. “Rare diseases are more common than their name implies; nearly one in 10 Americans has one. Yet fewer than 5 percent of the thousands of known rare diseases have treatments. So, there is a huge opportunity and a need for innovation.
“That said, the market is also segmented into very small populations of patients, each desperately waiting for a treatment. So, you have to move swiftly, pick areas of high potential patient benefit and value and, if you’re fortunate, ones where the science and learnings might even direct you toward effective treatments for other conditions.”
There’s also a delicate balance in managing needs and risks during R&D. The path toward regulatory approval is expedited and often uncharted, so teams have to learn to be comfortable with some level of ambiguity along the way. Data can emerge in unexpected ways, and you may have to develop new methods of testing for evidence you need to meet regulatory standards — as Enzyvant has had to do for RVT-802.
To operate successfully under these conditions, Enzyvant staff systematically and regularly analyzes and identifies areas of risk to the product or process and implements fixes to de-risk potential issues and routinely updates regulators on adaptations in the process in order to stay aligned with FDA expectations. The regular touch points and pressure tests allow for a measure of comfort with where things stand, confidence in direction, and ability to adjust rapidly when needed, which they say has been critical.
A LOT ACCOMPLISHED, AND QUICKLY
In just two short years, the company has built scientific and management teams, added two therapeutics to the pipeline, is submitting a rolling BLA, has worked with the FDA to develop a new assessment framework, and is working with payers to create a new reimbursement method.
“Alvin and the entire team have helped advance our R&D efforts, not only rapidly, but also creatively and thoughtfully,” says Jacques. “We are now poised, just a couple years in, to potentially see our first approval later this year.”
Enzyvant has been helped along the way by being part of the network of 15 companies (called “Vants”), created by Vivek Ramaswamy, founder and CEO of Roivant Sciences. These companies are structured to be nimble, operating autonomously, while sharing some resources (like access to talent) that help them quickly achieve meaningful therapeutic improvements.
WHAT’S NEXT
With the BLA filing for RVT-802 nearly across the finish line, Enzyvant is starting to consider alternative indications. It also is developing a second therapeutic, RVT-801, an enzyme replacement therapy for Farber disease, which is in preclinical development and has Orphan-Drug status in the U.S. and EU. The company plans to take these products all the way to commercialization.
Enzyvant’s leadership is convinced orphan drugs can be profitable, despite their small markets, and hopes to create a model that works for many one-time therapies in rare diseases.

