Preparing For A Blockchain-Enabled World
By Camille Mojica Rey, Contributing Writer
Follow Me On Twitter
Ask life sciences industry leaders and experts about blockchain, and you will hear it called everything from “a game changer” to “a major disrupter.” According to the hype, the technology behind cryptocurrencies, like bitcoin, is going to completely transform day-to-day operations for life sciences companies.
Blockchain technology, which allows the creation of shared digital ledgers, has been touted as a way to increase supply chain security, decrease human error and fraudulence in clinical trials, and improve postmarketing surveillance. And these are just some of the big-picture problems people expect blockchain to resolve. As blockchain technology is developed, few doubt that it will change the way data is collected and shared within companies, as well as between companies and third parties — such as CROs, partners, and regulators.
“Blockchain holds great promise for the life sciences industry,” says George Serafin, national managing principal of Grant Thornton’s Health Care and Life Sciences practices. A 30+ year veteran in the life sciences industry, Serafin was involved in writing a Spring 2017 research report, titled The Future of Growth and the Life Sciences Industry. The report called blockchain a “groundbreaking technology” that “can be leveraged for a variety of solutions across the life sciences value chain.” Serafin says industry leaders need to be clear about what blockchain is and what it is not. “The leadership of more companies needs to become better-educated with respect to blockchain technology. It’s not a silver bullet, and it requires significant investment.”
Pharmaceutical industry leaders agree. They know a blockchain-enabled world is on the way. A 2017 survey conducted by the industry nonprofit the Pistoia Alliance found that 83 percent of the 120 senior pharmaceutical and life science executives they asked expected blockchain to be adopted in less than five years. Still, only 22 percent of life sciences companies in the Pistoia survey were already using or experimenting with blockchain. “The Pistoia Alliance recognized early on that interest in blockchain among life sciences companies was growing rapidly,” says Nick Lynch, a consultant with the organization. As blockchain becomes more widely adopted for storing and sharing data in other sectors, the alliance is responding with educating their members through webinars and special sessions at their annual meetings. Yet their statistics show that the interest has yet to translate into use of blockchain. That’s likely because many questions remain unanswered for the average executive: 1) What exactly is blockchain? 2) How can it be applied, both within a company and industrywide? and 3) How do companies prepare for this blockchain-enabled world?
UNDERSTANDING BLOCKCHAIN
Fundamental knowledge is necessary if pharmaceutical industry leaders are to have a more accurate understanding of what blockchain can realistically do and in what time frame. However, the hype around blockchain has resulted in a phenomenon in which few people are willing to admit they don’t actually understand how it works. “Not many people understand blockchain enough to tell you what it can actually do,” says Daniel Himmelstein, Ph.D. Himmelstein is an expert in biological and medical informatics and a postdoctoral fellow in the Department of Systems Pharmacology and Translational Therapeutics at the University of Pennsylvania. “A blockchain itself is a data structure that allows its users to maintain a distributed database without having to trust each other,” Himmelstein explains. Currently, this structure, which can be thought of as a distributed ledger, is primarily used to enable financial transactions between participants, but can also be used to store small amounts of data or host programmable money, called smart contracts. Smart contracts are created so that the currency gains logic. For example, this could involve payments with expiration dates or payments that require approval from multiple entities.
One blockchain application encodes a unique hash, or digital fingerprint, of a document into a transaction, thereby time-stamping the document. This method can be used to prove that a PDF, such as a clinical trial protocol, existed at some time in the past. Instead of being stored on a single server, the ledger is stored at each node of the network. The nodes employ consensus protocols, which are the rules by which the network abides. Any new transactions are verified by the nodes. If the data entered do not follow the rules, the new transaction is not accepted. This means, for example, the hash of a time-stamped document cannot be changed later. “Consensus protocols adopted by the network provide security to make the blockchain immutable,” says Himmelstein.
Preservation by amber is Himmelstein’s favorite analogy for explaining how transactions become secure once placed on the blockchain. “Imagine that a fly becomes trapped in amber and every 10 minutes new amber forms around it. The amber gets larger and larger as time goes on. If you see a huge chunk of amber, you know it’s been there a long time.” The same goes for data placed on a blockchain. “It’s a well-accepted idea in computer science that bitcoin transactions placed on the blockchain are practically irreversible in just a few hours.”
The blockchain becomes resistant to change and deletion because it would take an unprecedented amount of computer power to rewrite a past transaction, explains Darryl Glover, a clinical pharmacist and YourEncore expert in the application of blockchain. “You would have to have huge, government-level computing power to change tens of thousands of copies at the same time,” Glover explains. The bitcoin blockchain has not been hacked since it was first introduced in 2009. There have, however, been a few instances of breaches in its security. These involved smart contracts that were attached to the blockchain. “You can’t update a smart contract. Any bug you have exists forever,” Himmelstein says. Smart contracts are a new area of computer programming. “At this point in time, it’s hard to create smart contracts without bugs because it’s such a new field.” Glover says vulnerability lies in anything external to the blockchain. “Company leaders need to be sure to limit this kind of exposure.” These breaches, combined with a perception that blockchain is too new to trust, have created some fear of the technology, Glover says. “Blockchain is not new. It’s based on cryptography invented in the 1940s and public/private keys developed in the 1970s.” What happened is that the people behind bitcoin combined these established technologies in a new and unique way. “There shouldn’t be any more fear about implementing the use of blockchain than there is over using Microsoft Office.”
BLOCKCHAIN APPLICATIONS
Blockchain works best when it is viewed as complementary to existing systems that generate data a company would like to time-stamp and share. According to Glover, “Blockchain is meant to facilitate, not replace.” He adds that blockchain is best used to:
- Build bridges between existing systems, internally or externally.Create true data provenance
- Know the type of data and be able to trace origin of data
- Create a network of trusted partnerships
![]()
"There shouldn’t be any more fear about implementing the use of blockchain than there is over using Microsoft Office."
Darryl Glover
YourEncore expert
Widespread acceptance of blockchain will require pharmaceutical makers to transition from the current process-oriented data structure where information about a pharmaceutical product is stored in the databases of every entity that comes in contact with it, from those who supplied ingredients to the pharmacy where the drug is dispensed. Instead, a productoriented data structure will allow all information associated with a product to be shared on a blockchain. (See Figure 1.) This transition will address a whole host of issues that plague the industry, says James Canterbury, EY global life sciences risk & compliance leader. However, blockchain will not be the answer to every problem, Himmelstein says. “What blockchain networks are good at is providing a new level of trust. You don’t have to trust a third party to verify the authenticity of something on the blockchain. This differs from the traditional model, especially in the pharmaceutical industry where almost every step has some level of trust in it.” Pharmaceutical companies have to trust they have purchased pure ingredients or that the CRO they have hired has not changed a protocol, for example. “Some of that trust can be removed by switching to blockchain,” Himmelstein says. Only things that are automatable can be switched to blockchain, such as verifying the authenticity of a digital document or tracing the provenance of a token representing pharmaceutical ingredients. “Those things that require human intervention will see less benefit.” Below are some of the areas thought to be the most likely to be revolutionized by blockchain networks.
FIGURE 1: PROCESS VS. PRODUCT ORIENTATION OF DATA STRUCTURES
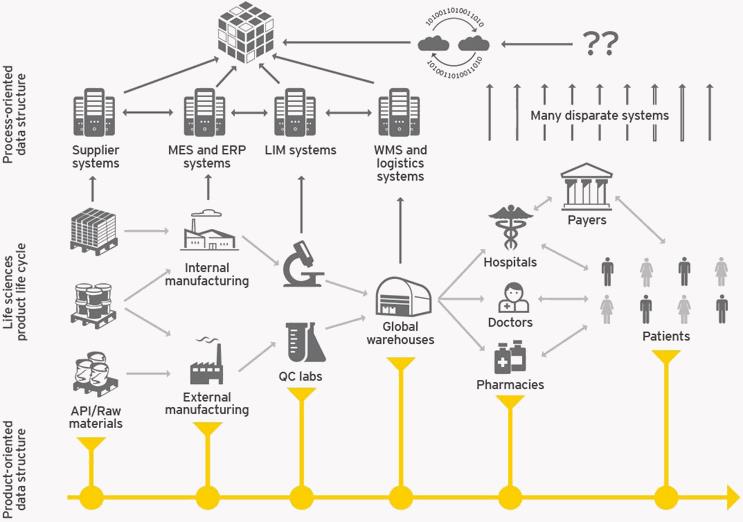
SOURCE: EY
Supply Chain Security. Experts agree that supply chain security is one of the best fits for blockchain technology. In fact, Canterbury says that if bitcoin had been around for 10 years instead of five, companies working to comply with the global serialization requirements that go into effect in November 2017 would likely be doing so with a global blockchain network. “Blockchain missed the boat in terms of timing, but may be a good Plan B for serialization.” Canterbury says bitcoin itself is still an experiment of sorts. “In life sciences, before anything becomes official, it has to be proven out extensively — which is a good thing — and that takes time.” Canterbury describes what a blockchain would look like for a pharma product in a white paper published in June 2017. Such a blockchain would start with batch creation and follow the product through to the smallest saleable unit. Because blockchains can refer to other blockchains, companies will likely begin with blockchains that collect data from processes that are within their control before including external manufacturers and distributors. According to Glover, counterfeiting in the U.S. happens primarily at the pharmacy level. “What blockchain creates is a single source of truth. You prevent someone from outside the system from inserting false numbers.” But, because most attacks come from an internal source, Glover says that biometrics should be married with private keys. “If you verify a person’s identity using retinal, finger, or facial scans at the time of making a transaction, there is no way anyone can say that someone took their key.”
Clinical Trials. Himmelstein says one issue that would be easily resolved by distributed ledgers would be the problem of the “professional patients,” people who volunteer for clinical trials — sometimes simultaneously — without disclosing participation to those running the trials. “The problem with clinical trials as they are now is that when patients enroll, you can’t track them from trial from trial.” Himmelstein says that at least one drug failed to receive approval because a percentage of the patients in the clinical trial were shown to be in multiple trials. A clinical trial’s blockchain would also store investigator data, allowing easy access to up-to-date credentials. Blockchain could also facilitate the transfer of information from clinical sites to CROs and manufacturers. Right now, moving data between systems is a time-limiting step. Most importantly, because of the immutable time-stamping, a blockchain network would improve overall data provenance for clinical trials.
Pharmacovigilance. Blockchain will give pharmaceutical companies the opportunity to proactively gather data. Right now, data gathering on adverse events is reactive. Glover points to the case of one Silicon Valley company that has developed a system that includes ingestible sensors, a small wearable sensor patch, an application on a mobile device, and a provider portal. This is the kind of data, as well as traditional survey data, that would be useful to store on a blockchain. “Now you can start looking at outcomes. If you survey actively, you can detect problems early and avoid an expensive, extensive recall. You can also go back to insurance companies and show that your drug has not required hospital admissions and patients’ risk factors are down, etc.,” Glover says. Patients would be rewarded for providing data because getting patient-level data and protecting identity is the ultimate goal. If a drug recall were to occur, that data could be matched to serial numbers and only those serial numbers linked to adverse events. “Blockchain could facilitate public safety, while saving drug companies millions in discarded product.”
PREPARING FOR BLOCKCHAIN
Pharmaceutical companies large and small are looking at how to prepare for blockchain. It is how they prepare that will make a difference, says Canterbury. “When people in the life sciences start looking into blockchain, they start looking at the industry’s big problems,” Canterbury says. However, big problems are going to involve large networks that require widespread participation across the industry. Instead, companies need to start by focusing on smaller, internal problems and the networks that already exist within a company. Every company, for example, collects quality metrics around batching. Switching the storage of that data from a database to a blockchain — or embarking on other internal pilot blockchain projects — has its advantages, Canterbury says. “First, companies will have more accurate and reliable ways of collecting internal data. Second, companies will develop teams with the skillsets and the understanding required to be on the forefront of industrywide blockchain applications without having to disrupt everything.”
The Pistoia Alliance is advising its members to begin looking for opportunities to collaborate. “Stakeholders — including life sciences companies, tech firms, and regulatory bodies — must begin a dialogue on the creation of industrywide data-sharing standards during this early adoption phase. These standards will improve security and render patients more likely to share their data with companies, benefitting everyone from researchers to patients, both now and in the future,” Lynch says. Because the organization’s mission includes encouraging collaboration, it already has a legal framework for sharing their members’ blockchain experiences in a safe, noncompetitive arena.
For the industry as a whole, significant barriers still remain to the widespread implementation of blockchain, Serafin says. One of those barriers is that companies have significant investments in their current platforms. Once leadership gets on board, there is still the challenge of migrating existing platforms to cloud-based solutions. “The IT departments are wrestling with this now,” Serafin says. The most important unknown is how the FDA and other global regulators will respond to blockchain’s potential. “Due to its highly regulated environment, the pharmaceutical industry is one that follows, instead of leads. It looks to regulators for the nod of approval. There has been no nod, yet.”
Sam Hume, Ph.D., expects that nod to come. “Blockchain may well be next,” says Hume, head of Data Exchange Technologies at the Clinical Data Interchange Standards Consortium (CDISC), a nonprofit that develops data standards to foster smarter research and enable connections to healthcare. “Blockchain promises to solve some thorny problems in the industry, especially for regulators. It’s too early to say we’re working on it, but as our members start to do more development work around blockchain, and as the technology matures, we will work with them to figure out how to make our standards work with their technology.” Companies that perform regulated clinical trials are required by the FDA and PMDA (Japan) to submit data using CDISC standards. Increasingly, nonregulated researchers are adopting these standards, as well. This is important because the need for standardization will only increase in a blockchain-enabled world. Hume predicts the widespread adoption of blockchain will be much like what took place when the internet was first introduced. “It took about a decade for web to scale up. It just takes longer when things require cooperation.” The transition to blockchain-based systems will be painful at first. “We won’t really see the full benefit of the technology until the large blockchain networks are in place.”

