Research Reveals Critical Need To Eliminate Clinical Silos
By Jim Reilly, VP of clinical market strategy, Veeva System
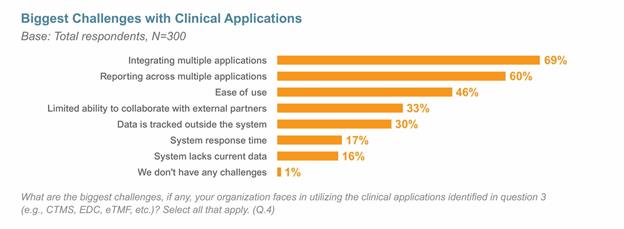
 New research confirms that many of the challenges sponsors face managing clinical trials stem from the disparate nature of processes and systems. The Veeva 2017 Unified Clinical Operations Survey, one of the industry’s largest surveys of clinical operations professionals, indicated that nearly all respondents cited the need to better integrate their clinical applications, including EDC (electronic data capture), CTMS (clinical trial management systems), and eTMFs (electronic trial master files). Faster study execution and improved study quality were the top drivers in bringing together end-to-end processes and systems.
New research confirms that many of the challenges sponsors face managing clinical trials stem from the disparate nature of processes and systems. The Veeva 2017 Unified Clinical Operations Survey, one of the industry’s largest surveys of clinical operations professionals, indicated that nearly all respondents cited the need to better integrate their clinical applications, including EDC (electronic data capture), CTMS (clinical trial management systems), and eTMFs (electronic trial master files). Faster study execution and improved study quality were the top drivers in bringing together end-to-end processes and systems.
The survey showed that it is common for companies to use as many as four or five applications to manage their clinical studies, with EDC and CTMS being the most commonly used applications. The top two challenges cited — integrating multiple applications and reporting across applications — are a direct result of system silos.
STUDY START-UP CHALLENGES
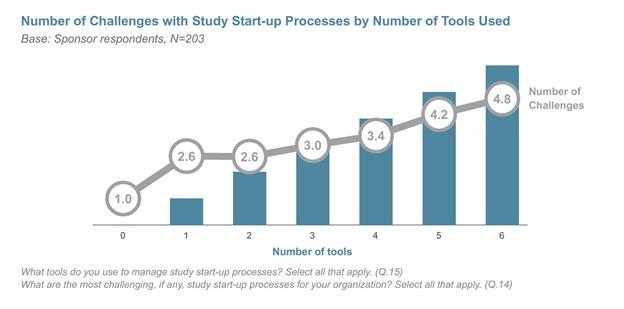
Both the Veeva study and a separate report from Tufts Center for the Study of Drug Development found that approximately 95 percent of sponsors report challenges with study start-up — usually due to the use of multiple systems and tools. For example, the vast majority still rely on spreadsheets to manage the study start-up process, with roughly one-third or less using CTMS, eTMF, or home-grown applications. Companies that use two or more tools to manage study start-up practices report encountering at least three challenges.
Both studies also found that the time from the pre-study visit to contract execution accounts for the majority of the lengthy study start-up cycle time. Almost two-thirds of sponsors in Veeva’s survey say contracting and budgeting are their most challenging study start-up processes. Site identification selection and study planning during protocol design were the next most oft-cited challenges.
MODERN SYSTEMS ARE IMPROVING CLINICAL PROCESSES
New applications and platforms are helping life sciences companies streamline the clinical ecosystem. Trial master file (TMF) management solutions, for example, have experienced rapid transformation during the past four years, with one in three sponsor companies now using advanced eTMF applications, more than doubling since 2014. And 77 percent of sponsors using modern eTMF applications report improvements in inspection readiness and managing the growing volume and complexity of clinical trials.
Similarly, there is also a shift underway with CTMS. A Markets and Markets report forecasts life sciences organizations will increase their CTMS investments by almost 15 percent each year through 2020 as sponsors report significant deficiencies in their current systems. This is being driven, in part, by rising demand for data and site-collection solutions and the greater availability of next-generation CTMS applications.
THE RIPPLE EFFECT
Clinical trials are a critical part of the broader product life cycle, including regulatory, quality and manufacturing, commercial, and medical. As the next big step forward, information will flow through to other parts of the organization and have a positive impact across many areas.
Clinical and other functional groups need to access much of the same data during different stages of drug development. Rather than using redundant, manual processes or complex integrations, cloud-based platforms enable different teams to access the same information in multiple ways.
As the research shows, there is a tremendous opportunity for companies to transform their operations by streamlining their clinical environments. Doing so will undoubtedly drive new levels of efficiency and effectiveness across the entire clinical ecosystem.
