Scaling Cell And Gene Therapy Manufacturing Operations
By Sanjay Srivastava, PhD, Managing Director, Cell & Gene Therapy CoE Lead; Boris Bogdan, Managing Director, Oncology CoE lead; Sandra – Dietschy-Kunzle, Senior Principal, Oncology CoE; and Benjamin Ferrara, Strategy Manager, Life Sciences at Accenture
This is a two-pronged approach to address scarcity of resources.
As with any complex process, cell and gene therapy (CGT) manufacturing carries its own distinct challenges. Current  approaches to address these challenges only focus on certain aspects of the process. The life science industry must adopt new approaches to manage the high cost of goods sold (COGS) and cope with the significant rise in capacity need.
approaches to address these challenges only focus on certain aspects of the process. The life science industry must adopt new approaches to manage the high cost of goods sold (COGS) and cope with the significant rise in capacity need.
Manufacturing autologous cell therapy products faces few key complications, which is typically why CAR-T manufacturing runs at two or three sigma operations. First, individual patients are the source of raw material cells, and every patient is different, creating broad variability in extracted patient cells. The quality of the final product is closely tied to the biology of an individual patient’s cells. This introduces unpredictability in a recipe’s key ingredients and in the manufacturing process cycle time.
Second, many CGT manufacturing processes have been developed based on relatively small patient populations, primarily through a manual process. These exceedingly manual manufacturing processes leave room for broad variability in the operations, because each operator executes the process differently. The very nature of human variability introduces more risk and complications into the final product specifications themselves.
Third, CGTs rely on biological starting material with shorter shelf lives and greater temperature sensitivities. There are also increased complexities and costs tied to purity and identity testing. The supply chain for autologous cell therapies is therefore complicated and requires proximity to the patient during the manufacturing process.
Life sciences companies have taken at least three approaches to solve these manufacturing challenges:
- Increased footprints. To drive the innovation engine, tighten the supply chain and help establish an expansive manufacturing footprint, life sciences companies are building hub and spoke models. Companies are decentralizing their operating models by moving the value chains closer to treatment centers and patients.
- Increased resources. CGT companies are unable to take advantage of economies of scale and build out by investing in a broad array of talent and equipment. However, the need for talent outweighs the current capacity of the talent pool, and equipment procurement has long lead times and is not cost effective.
- Serial, closed loop systems and automation. There is an increasing trend in the deployment of a closed system — automated and scalable for cell manufacturing procedures—from starting material to final cell product. The closed system helps eliminate open processing steps and reduces operator transfers between multiple pieces of equipment. These practices have minimized the use of paper but increase reliance on automating process steps and implementing electronic batch records. While manufacturers engaged in such automated processes are experiencing consistency in operations, relatively lower quality deviations and shorter product release cycle times, the scale remains elusive.
What To Do?
The current tactics in CGT manufacturing are noteworthy and have been a key factor in driving huge improvements for the first generation of operations. But they are insufficient to ensure that the industry will have enough capacity at reduced prices by significantly bringing COGS down and increasing market access to drive CGT adoption.
Current efforts are focused on improving operations by developing and implementing technologies that can help optimize onsite manufacturing processes. We believe there must be step change in the approach to scaling up, while properly managing COGS. The industry should continue increasingly adopting new technologies and supplementing them with fundamentally new approaches to optimize CGT manufacturing.
We believe that we must employ two levers — on a local and holistic level — to improve the use of manufacturing assets and drive scale. This two-fold strategy entails focusing on the individual sites and on the network as one system — the sum of all available capabilities.
Figure 1. Optimizing CGT Manufacturing
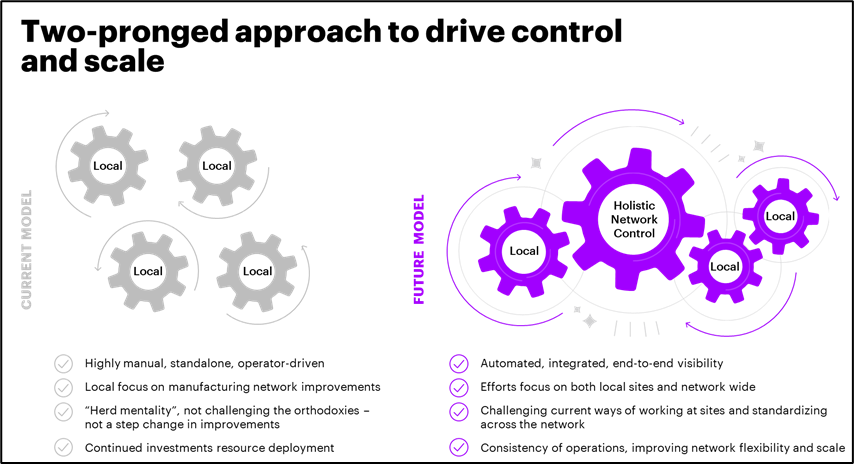
Focusing Locally
At individual manufacturing sites, we need to challenge the current orthodoxy on scale-limiting, laboratory-based, single, serial manufacturing processes in a manufacturing suite. Current cell therapy manufacturing techniques call for a set of equipment organized serially to complete a linear process. However, this linear system limits equipment variety and introduces bottlenecks in the critical path. A majority of process improvement efforts have focused on optimizing the current linear setup.
Beyond creating process automation and digitization, we need to introduce parallelism and modularity, and create a process control infrastructure to improve increased scale, predictability and consistency. The parallel equipment setup will offer greater scalability by helping manage various productions simultaneously. Further, modular manufacturing can facilitate process variations to accommodate different modalities and enable multiple copies of the equipment to remove bottlenecks in the process.
The process control infrastructure will enhance data visibility, reducing the time required for data gathering, reporting and analyses of process performance. However, implementing parallel and modular systems requires the industry to challenge its current linear setup and highly manual, operator-driven process to adopt more automated and capital-intensive operations, but at relatively lower operational costs.
Focusing Holistically
Under traditional biologics, approximately 35% of the manufacturing process is outsourced, whereas cell and gene therapies outsource 65% or more of their manufacturing processes. Life sciences companies are building hub and spoke models to establish a manufacturing footprint. Manufacturers have deployed strategies closer to the patient with Day 0 cell processing and drug substance processing for final formulation of gene therapies. Maintaining centralized cell and tissue manufacturing closer to the markets where highly specialized CGT manufacturing capabilities already exist provides certain advantages.
However, organizations are either unable to scale or lack agility across the manufacturing network. Manufacturers engage with each partner as a standalone entity, restricting their view of the entire manufacturing network as one complete system. Data integration between the internal network and contract manufacturing organizations is primarily manual, with limited or no automation for data transfer. Under this model, there is no end-to-end visibility to enable effective production planning or decision-making for optimal production opportunities to leverage capacity across the network.
With more than 200 parameters on process characterization (continuous process verification, investigation, regulatory, process data), establishing a standardized site level infrastructure and equipment deployment and automated data integration infrastructure will facilitate manufacturers’ ability to respond rapidly to production changes and needs across the network. The consistency of operations will reduce operator and site variability, improving network flexibility and response time in case of failures.
Because manufacturing engineers and researchers will spend less time manually collecting, transcribing and verifying data, such an infrastructure will lead to improved productivity and provide a foundation for deeper scientific investigation and data sharing.
Beyond Manufacturing Improvements
With escalating demand for CGT, farsighted improvements within manufacturing are imperative. However, it is not enough to improve the efficiency of the manufacturing process alone. Instead, life science organizations need to take both a more local approach with the goal of improving processes at individual manufacturing sites and a more holistic view aimed at better coordinating the different production steps within the network.
Ultimately, the key challenge is neither simply technological nor organizational. It is committing to — and investing in — a rapidly developing field by taking a fresh path.
To manage the uncertainty around CGT manufacturing, improving processes means building in flexibility with optionality and experimentation around a modular but stable backbone. Taking this approach to balance risk and innovation will ultimately result in decreasing manufacturing costs while ensuring quality, making this technology scalable and more accessible.
Increasingly, CGT (cell and gene manufacturing) is proving to be a field worth investing in; this is the promising future of therapeutics.
The authors would like to acknowledge Praveen Govindrajan, who provided useful insights for this article.


