Science Does Not Sell Itself: Designing A Winning Pivotal Trial
By Simone Seiter, Kevin Baruzzi, and Christian Hinneburg

In the world of biopharmaceutical innovation, groundbreaking scientific discoveries often do not directly translate into commercial success. Simply developing a promising therapeutic based on great science is not enough. For science to become a winning product, three critical steps must be mastered: selecting the right indication, designing a winning pivotal trial, and defining a winning commercialization strategy. Among these, pivotal trial design is perhaps the most crucial, as it can make or break a product’s commercial viability. Even with a strong asset and a high unmet medical need in a particular indication, the wrong trial design can severely limit both the peak patient potential and the price potential of the therapy.
This article will focus on the second step: Designing a pivotal trial that positions a drug for commercial success. Specifically, it will explore how choosing the right patient population, endpoints, and comparator are critical for achieving a favorable outcome and maximizing the market potential of a therapy.
The Three Components Of A Winning Pivotal Trial
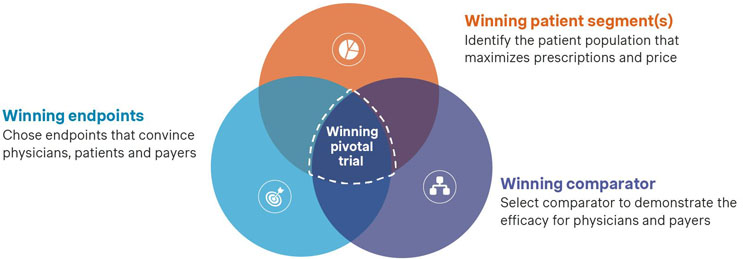
Choosing A Winning Patient Population (Component #1)
Selecting the right patient population is a critical aspect of trial design, and the approach can vary significantly depending on the therapy and its commercial objectives. In some cases, narrowing the patient population to a specific segment can sharpen the efficacy signal, leading to a more compelling case for the therapy’s effectiveness. In other cases, broadening the population may better capture the full potential of the therapy, ensuring a larger patient base. The decision of whether to narrow or broaden the population depends on the unique characteristics of the drug, market specifics, and consultations with regulatory agencies.
For example, a biotech company initially planned to include patient segments A, B, and C in its pivotal trial. However, after analyzing efficacy data, it became evident that segment B was better suited for demonstrating a strong efficacy signal. By narrowing the focus to this segment, the therapy’s potential for higher pricing and stronger market uptake was enhanced. A strong efficacy delta — greater efficacy relative to the comparator — translates into both higher patient potential and higher price potential. Particularly in regions like Europe, a smaller efficacy delta can lead to diminished price expectations, along with slower uptake, limiting the therapy’s overall commercial potential.
Another example involves a biotech company developing a gene therapy. The company originally planned to focus on a specific indication for its pivotal trial. After conducting a thorough analysis, a shift to a mutation-specific design was recommended rather than an indication-specific one. This strategy can broaden patient potential, as past gene therapy approvals have shown that regulatory labels are often mutation-specific rather than indication-specific. By focusing on the specific mutation, the therapy could be positioned more effectively to target a larger patient population.
By carefully assessing whether to narrow or broaden the patient population — through consultation with regulatory agencies and key stakeholders — companies can maximize the patient potential of their therapy.
Choosing Winning Endpoints (Component #2)
Selecting the right endpoints can make or break a trial. Endpoints that resonate with healthcare providers (HCPs), payers, and patients alike are essential for success. While clinical endpoints — such as biomarkers — may be commonly used, payers as well as HCPs are rather focused on functional endpoints, which measure the impact of the drug on a patient’s life.
In one case, a biotech company initially chose an endpoint that was well-accepted by KOLs (KOLs) in the field to measure disease progression. However, after consulting with a broader group of stakeholders, including HCPs and payers, it became clear that these groups preferred an endpoint that more directly demonstrated improvements in patients' lives. Shifting from the KOL-preferred clinical endpoint to a functional endpoint not only increased the likelihood of favorable price potential but also made it easier for HCPs to explain the therapy’s benefits to patients.
Selecting A Winning Comparator (Component #3)
Choosing the right comparator is another critical factor, as selecting the wrong one can result in a diminished commercial potential. A therapy that appears less effective than it truly is due to an inappropriate comparator may struggle in the market, as healthcare providers and payers are less likely to adopt or pay a premium for it.
In one scenario, a biotech company planned to compare its novel therapy with best supportive care (BSC) — the only approved option for the indication. However, in practice, HCPs were using an off-label therapy (therapy C). By switching the comparator to therapy C, the trial design was aligned with real-world clinical behavior. This choice was crucial: Without comparative data against therapy C, HCPs signaled they would be reluctant to prescribe the new drug, and payers indicated they would cap the drug’s price at or below the price of therapy C. Selecting the correct comparator ensured the therapy’s commercial and clinical relevance.
Why Trial Design Matters For Commercial Success
The design of a pivotal trial is a key determinant in the commercial viability of a new therapy. Ceteris paribus (all other factors remaining the same, such as the asset and the indication), the wrong trial design can drastically undermine the potential success of a drug. The choice of patient population, endpoints, and comparator directly impacts the therapy’s perceived efficacy and value in the market, ultimately affecting both its peak patient potential and its price potential.
For example, selecting an overly broad or inappropriate patient population can dilute the efficacy signal, leading to slower uptake and lower price potential. Conversely, a narrow patient population could highlight stronger efficacy but might limit the therapy’s market potential if it excludes key segments. There is no one-size-fits-all approach; the optimal patient population depends on the unique characteristics of the asset and the indication. The decision to narrow or broaden the patient population must be carefully evaluated, considering both the clinical potential and the commercial objectives of the therapy.
Similarly, choosing the wrong endpoints — those not aligned with payers', HCPs’, and patients' expectations — can reduce the perceived impact of the therapy on patients' lives, affecting the therapy’s uptake and price potential.
The choice of comparator is equally crucial. Failing to align the trial with real-world clinical practices can result in data that stakeholders, such as healthcare providers and payers, do not find compelling. This misstep can lead to slower market adoption and price caps, regardless of the therapy’s actual clinical benefits.
Even with a promising asset and a high unmet medical need in the selected indication, a poorly designed trial can severely limit a therapy’s peak patient potential and its price potential. On the other hand, a well-designed trial, tailored to stakeholder expectations, can ensure that the therapy is positioned for strong market uptake, premium pricing, and long-term commercial success. Understanding these factors early in the design process and engaging in stakeholder consultations, including regulatory agencies, are critical steps to avoid costly mistakes and ensure the trial sets the stage for commercial viability.
Holistic View Of Trial Design Needed
In summary, designing a winning pivotal trial is a complex balancing act. To ensure commercial viability, the right patient population, endpoints, and comparator must be carefully selected. Each decision has far-reaching implications for the drug’s market positioning, pricing, and adoption rate. By taking a holistic view of trial design that incorporates scientific, clinical, and commercial perspectives, biopharmaceutical companies can transform groundbreaking science into a winning product that changes lives.
A winning trial design is one of the crucial steps, alongside selecting the right indication and crafting a winning commercialization strategy, that can ultimately sell the science. While great science is the foundation, it is the combination of these three elements that turns a promising therapeutic into a successful product. Science alone cannot sell itself: A winning trial design, when integrated with the right indication and commercialization strategy, can.
 About The Authors:
About The Authors:
Simone Seiter is a Senior Partner at Simon-Kucher based in Frankfurt, Germany, and the Head of Commercial Strategy Pharma & Biotech. She has worked within the global biopharmaceutical industry for over 20 years and has deep expertise in strategy, portfolio management, and commercialization.
 Kevin Baruzzi is a partner in Simon-Kucher’s Healthcare and Life Sciences division based in their Boston office. He primarily supports pharmaceutical and biotech clients, with particular areas of expertise include new product planning, launch and in-line brand strategy, and product/portfolio growth strategy.
Kevin Baruzzi is a partner in Simon-Kucher’s Healthcare and Life Sciences division based in their Boston office. He primarily supports pharmaceutical and biotech clients, with particular areas of expertise include new product planning, launch and in-line brand strategy, and product/portfolio growth strategy.
 Christian Hinneburg is a Senior Manager at Simon-Kucher in Cologne, Germany, with a focus on commercial strategy in pharma and biotech.
Christian Hinneburg is a Senior Manager at Simon-Kucher in Cologne, Germany, with a focus on commercial strategy in pharma and biotech.
