The Ozempic Dilemma: What Makes A Dual Brand Approach Viable?
By Andrew Parece and Kris Vezuli, Charles River Associates
Recently, there has been heightened focus on dual brand approaches in which the same molecule is approved for multiple indications and packaged, priced, and distributed under different brand names. Public interest has centered on Novo Nordisk’s semaglutide, with its dual brands Ozempic1 (type 2 diabetes) and Wegovy2 (weight loss), both once-weekly self-administered subcutaneous injections (a third brand of semaglutide, Rybelsus, is an oral formulation administered once daily). Ozempic and Wegovy are available with different dosage strengths (Ozempic in three strengths, Wegovy in five) and at different prices per pen and per unit. Ozempic’s monthly list package price is approximately $969 compared with Wegovy’s approximate $1,349.3 Given relatively limited differences in dosage strengths and labeled dosing for the two brands, the price difference has led to many patients requesting, and providers prescribing, Ozempic for weight loss. In addition, many payers have applied utilization management through prior authorization criteria to limit Ozempic use to patients with type 2 diabetes, as they typically would not reimburse for weight loss medications. Dual branding of semaglutide, with high-profile celebrity testimonials, has shone a light on dual brand benefits and complications as patients attempt to minimize out-of-pocket costs. Recently, Eli Lilly received approval for its dual-brand medication Zepbound (tirzepatide injection), a once-weekly therapy for weight loss, following its 2022 approval as Mounjaro for treating type 2 diabetes.
The follow-on indications associated with a second or dual brand often have significant differences in dosing, dosing frequency, formulation, route of administration, site of administration or other factors that may provide improved efficacy, safety, convenience, or other benefits. Similarly, dual brands often address different levels of disease burden and unmet needs associated with different patient conditions or prevalence.
Disease burden and unmet need drive value
One key factor affecting the viability of a dual brand approach is the need for different product versions to address underlying patient conditions. To the extent that a new indication for an existing therapy addresses a distinct patient population, then a separate brand may be appropriate. Most commonly, this situation is accompanied by different dosing requirements (strength, frequency, or titration) and route of administration or formulation, which are needed to achieve effectiveness in the new population due to the underlying differences in the disease. Even if significant dose volume adjustments are necessary to effectively treat the new condition with a different route of administration and/or dosing, the imputed price (at parity price/unit with the first indication) may not be sufficient to realize the difference in value in the new indication due to higher disease burden, unmet need, or efficacy. The pricing of a second brand may be set separately to reflect this distinct value. When dosing differences are negligible or simple to apply (e.g., double, or half the dose, with same frequency and route of administration), a single brand approach is likely to be more appropriate.
Table 1: Selected FDA approved dual brands
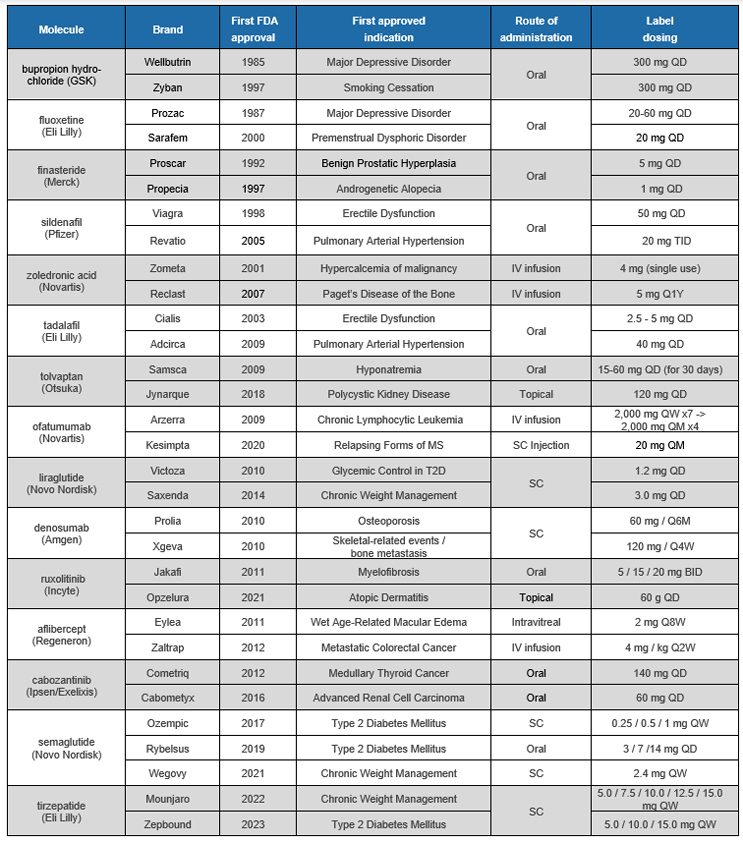
Source: CRA Research and Analysis, 2023
Factors driving differentiation
The most common differences in product attributes associated with consideration of a dual brand approach are listed in Table 2 below. All these differences are not required for a dual brand to be viable; conversely, several of these factors together does not necessarily mean that a dual brand approach will be effective. With each situation, the combination of factors and circumstances will dictate the appropriate approach.
Table 2 – Factors driving differentiation and dual brand potential

Source: CRA Research and Analysis, 2023
A dual brand approach is more viable when there is some significant product differentiation which corresponds with disparities in effectiveness or value across indications. For example, differences in dosing levels and frequency, loading dose or maintenance doses, route of administration (oral tablet/suspension, IV, subcutaneous injection, inhaled), and molecular composition (e.g., amorphous vs. crystalline) may support a dual brand approach. Similarly, some patient conditions may require HCP administration, physician-office administration or first-dose observation to address adverse reactions or side effects associated with some formulations. In some cases, safety issues associated with different dosing and formulations, and potential for patient or HCP confusion, could dictate dual brands.
Dual brand case studies
There are two common situations that can suggest or require dual brands: (1) significantly different dosage strengths are required across indications, and (2) formulation or route of administration differences exist for different indications. The following real-world examples illustrate the situations wherein dual brands with separate pricing may be viable, and conversely the conditions in which limited differentiation precludes a dual brand approach.
Dosage strength differences
Prolia and Xgeva (Amgen) are the two brands of denosumab, a RANKL inhibitor that works by decreasing osteoclasts that lead to degradation of bone:
- Prolia (2010) is indicated to treat osteoporosis in patients at a high risk of fracture. It is also indicated to reduce bone loss in breast and prostate cancer patients.4 The majority of Prolia patients are osteoporosis patients.
- Xgeva (2010) is indicated for the treatment of a significantly smaller patient population, i.e., for the prevention of SRE in solid tumor and multiple myeloma patients, giant cell tumor of bone, and hypercalcemia in patients refractory to bisphosphonate therapies.5
The dual brand approach for denosumab is based on dosing differences – Prolia is administered at 60 mg every six months whereas Xgeva is administered at 120 mg once every four weeks. Although Prolia is less expensive than Xgeva on an annual basis, the cost of Xgeva per mg is lower than Prolia. While using Xgeva in osteoporosis patients may seem less expensive on a per mg basis, Xgeva is supplied in 120 mg single-use vials whereas Prolia is supplied in 60 mg prefilled syringes or single-use vials, thus using Xgeva for osteoporosis may result in waste. Most payers do not appear to be managing Prolia and Xgeva differently, with a prior authorization to label typically required for both brands.
Formulation / Route of Administration (RoA) differences
Jakafi and Opzelura (Incyte Pharmaceuticals) are two brands of the molecule, ruxolitinib, a JAK inhibitor:
- Jakafi (2011) is indicated for three rare indications: myelofibrosis, polycythaemia vera, and acute and chronic graft vs. host disease (GvHD).6
- Opzelura (2021) was launched for atopic dermatitis refractory to topical treatments and has since expanded its indications to include vitiligo.7
The dual brand approach for ruxolitinib is based on a difference in formulation/RoA, as Jakafi is an oral medication whereas Opzelura is a topical treatment. There is also a significant difference in the prevalence of myelofibrosis and atopic dermatitis. The estimated annual price differential between Jakafi and Opzelura is about 96% with Jakafi being almost twice as expensive on an annual wholesale acquisition cost (WAC) price-per-patient basis. Jakafi is largely covered with prior authorization to labeled indication across payer plans. Opzelura is typically covered with a prior authorization to label for atopic dermatitis refractory to topical treatments; however, it is covered with restrictions for vitiligo where other topical treatments (steroids and calcineurin inhibitors) are often used as step therapy in clinical criteria for coverage.
Table 3: Prolia / Xgeva (denosumab) and Jakafi / Opzelura (ruxolitinib) dual brands8

Source: CRA Research and Analysis, 2023
Differential value and price alignment with dual brands
In some cases significant differences in dosing can lead to differences in cost across indications, if cost is imputed on a parity cost-per-unit basis. For example, when an existing product shows unique or exceptional effectiveness in a new indication that has a significantly higher level of disease burden or unmet need, and fewer therapeutic options, the value and cost-effectiveness of the therapy could be significantly higher in the new indication. With single brands, and single price per unit, there is limited ability to align price with value across indications (note: differential rebating and other value-based approaches can be applied to align net cost with value). However, a dual brand approach allows cost to be aligned with value as price per unit across the brands can be de-coupled. Figure 1 below illustrates how price can be aligned with clinical value when a second brand requires a different total dose (e.g., 50 mg vs. 100 mg for first brand) and provides greater clinical value.
Figure 1: Dual brand price per treatment based on clinical value

Source: CRA Research and Analysis, 2023
Conclusion
Increased use of dual brands by pharmaceutical manufacturers has been accompanied by scrutiny of the need for dual brands in specific situations. Without tangible differences in dosing, formulation, route of administration or other factors that relate specifically to a brand’s improved effectiveness or patient benefits in a new indication, a dual brand approach may not be viable. But when such differences are clear, and supported by evidence such as clinical trials, PK studies, and patient reported outcomes, a dual brand approach is more likely to be viable. In some cases, the volume of active ingredient of the second brand needed to achieve effectiveness may imply pricing that is not aligned with its value (at the price/unit of the existing brand), warranting different price per unit between the brands. Manufacturers will need to support the dual brand strategy and pricing with evidence, and clearly articulate the rationale for the dual brand and its cost.
About the Authors
Andrew Parece, Vice President
Andrew Parece has more than 30 years of consulting experience, and specializes in competitive strategy, pricing, quantitative analysis and market research in the pharmaceutical, biotech, and life sciences industries. He has managed competitive assessments and product launch strategy engagements for several high-profile products and franchises. He earned an MBA from Cornell University.
Kris Vezuli, Associate Principal
Kris Vezuli has over 15 years of experience in drug development and commercialization across a broad array of therapy areas, including autoimmune and inflammation diseases. He has expertise in creating value throughout the product life cycle and his background includes leading and managing projects in pricing and market access, commercial strategy, new product planning, strategic segmentation, and life cycle management. Kris earned an MBA from Babson College.
The views expressed in this paper are those of the authors and do no represent the views of Charles River Associates.
References
- Incyte Corporation. Jakafi (ruxolitinib). [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf. Revised September 2021. Accessed September 9, 2023.
- Incyte Corporation. Opzelura (ruxolitinib). [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309Orig1s000correctedlbl.pdf. Revised September 2021. Accessed September 9, 2023.
- Incidence is indicated by “per year”; Dosing is label dosing for maintenance; WAC = wholesaler acquisition cost; RoA = route of administration
- Amgen Manufacturing Limited. Prolia (denosumab) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125320s5s6lbl.pdf. Revised September 2011. Accessed September 9, 2023
- Amgen Manufacturing Limited. Xgeva (denosumab) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf. Revised June 2013. Accessed September 9, 2023
- Novo Nordisk A/S. Ozempic (semaglutide) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf. Revised December 2017. Accessed September 9, 2023
- Novo Nordisk A/S. Wegovy (semaglutide) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215256s000lbl.pdf. Revised June 2021. Accessed September 9, 2023
- Redbook. Accessed January 3, 2024
