Trust In Institutional Practices Keeps Pandemics From Becoming Pandemonium
By Suresh Kumar

An outbreak becomes an epidemic or pandemic for one reason: unavailability of medicine, vaccines, and procedures to treat or prevent the disease. Containing outbreaks until a treatment or prevention is found requires educating and securing compliance to appropriate social and cultural behavior and hygiene practices. Doing so requires leaders to set an example that influences and inspires the public. The U.S. falls short on each of these needs. That’s why the country accounts for 25 percent of the world’s infections and 22 percent of the worldwide COVID-19 fatalities. Its disappointing trajectory today contributes 38 percent of the world’s active cases and 27 per- cent of its serious cases.
The bottom line to managing pandemics is clear government protocols and public confidence in the administration and its public health institutions. Instead of building trust, the Trump administration has consistently undermined its institutions and pushed unproven theories over science. There is no greater risk to human health than a president bullying federal agencies to authorize scientifically unproven products or interventions. This is why America braces for “October surprises,” and it is why a majority of Americans say they are unwilling to take a vaccine approved by this administration.
OMINOUS FACTS AND TIMELESS PRINCIPLES
Coerced by the President, on March 18, the FDA approved chloroquine (CQ) and hydroxychloroquine (HCQ) without the science to back up its decision. On June 15 it reversed course, determining these products to be neither safe nor effective. Patients on this regimen for the interim 79 days risk “ongoing serious cardiac adverse events and other serious side effects.” How many patients were treated with CQ and HCQ, and, of those, how many experienced serious adverse events?
The ominous signals of October surprises appear to have started in August. On the opening night of his convention, the President, in an effort to report re- sults more favorable to the administration,
- again pushed hydroxychloroquine on national TV
- touted a conveniently timed FDA Emergency Use Authorization (EUA) for convalescent plasma therapy (CPT) (the government’s top scien- tists disapproved, requiring the FDA to secure more rigorous, robust, and structured studies than the two small, noncontrolled ones on the basis of which the authorization was granted).
The President is reportedly considering fast tracking a COVID-19 vaccine so that an EUA is granted by his administration (before the country goes to the polls) based on results of a 10,000-subject U.K. study and not the 30,000-subject U.S. study that is ongoing.
Urgency to announce untested and robustly vetted remedies risks lives. Theory must not trump science to compromise safety and effectiveness in approval of medical treatments, products, and protocols. Until the government proposes and gains approval for an improved drug approval process, the FDA must remain agnostic to politics and ensure integrity of the drug approval protocol that protects American citizens.
Protocols borne out of good policy, such as ones to bring drugs to market, endure as guideposts for a reason. They save lives. They require a disciplined and transparent stage-gate approach from preclinical to Phase 3. Governments, industry, and public health institutions working together to streamline a regulatory process is one thing, but circumventing the need for results borne of robust, double-blinded, placebo-controlled studies that pass peer review muster is completely another. How can public health institutions be shielded from political interference, presidential bullying, and pitting institutions against each other (e.g., HHS & CDC, NIH & FDA, BARDA & CDC, and HHS & CMS)? Doing so corrodes public health institutions whose only allegiance must be to the public and not politics.
POLICY BUILDING BLOCKS
Infectious disease outbreaks are becoming more frequent — there have been seven in the last 20 years. Pathogens develop resiliency to drugs and require institutions to remain a step ahead with superior science to combat new mutations and strains. While the next outbreak may not be predictable, cohesive policies can prepare us to combat future outbreaks and disease transmission trajectories.
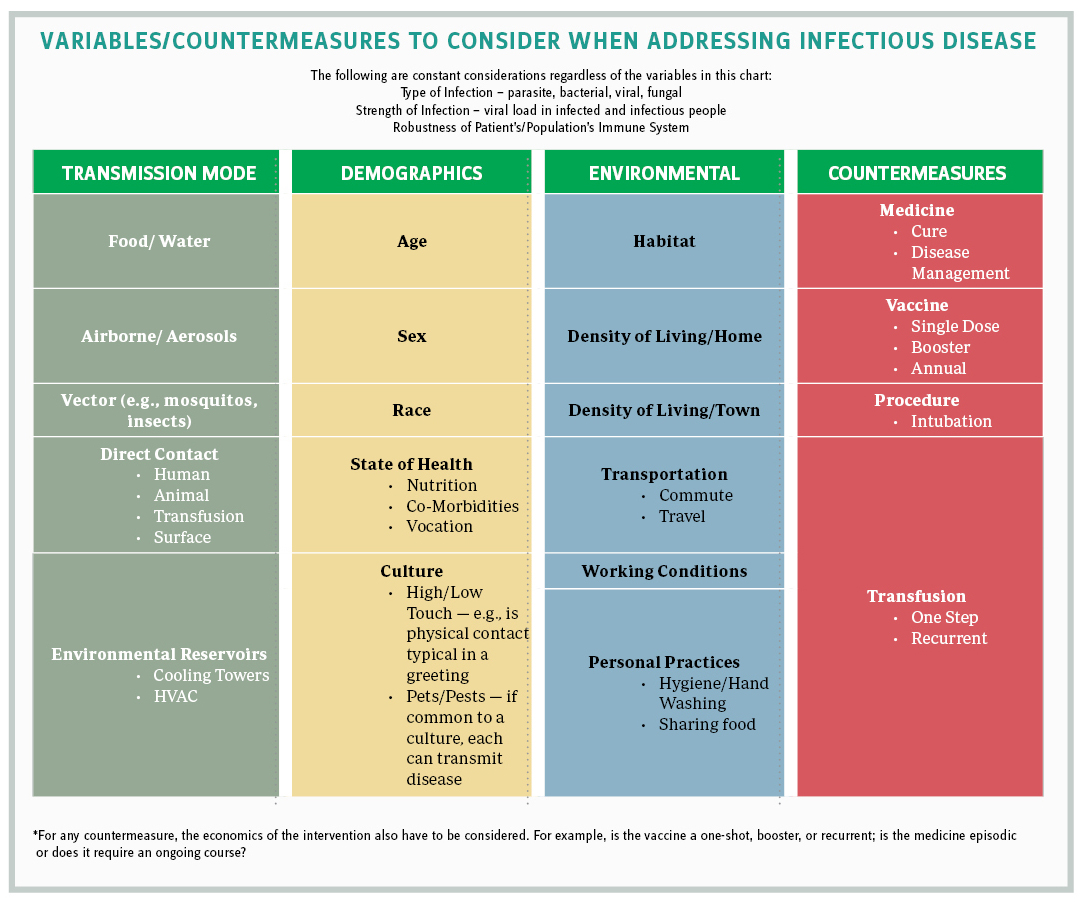
Understanding the building blocks of outbreak management helps us when developing policies that enhance our readiness to complete the final stages of clinical trials and to rapidly bring medical countermeasures to market. Having this knowledge makes us more prepared; for example, knowing when to build national stockpiles and how to tap resources to contain outbreaks (e.g., PPE, hospital beds, medical equipment, social protocols). Adjusting interventions to demographics and environmental variables helps to establish transparent, population appropriate, and effective supply chains. An annual public health budget of $1 billion and public-private partnerships can underwrite commercial risks inherent in preparing for the future and encourage timely commercial engagement and enhanced public safety.
Where is the U.S. government’s cohesive policy to combat outbreaks in the future or even for COVID-19 today?
It is worth noting that learnings from Ebola helped establish the WHO R&D Blueprint, a strategy and preparedness plan that prioritizes diseases and improves readiness to tackle an unknown “Disease X,” as the WHO calls it, through rapid activation of R&D activities during epidemics. It is the foundation that influenced the rapid access to and rollout of WHO COVID-19 testing products, supplies, and protocols, for which there was acute global need. The U.S. chose to pass on this and suffered deadly consequences.
POLICY ON TESTING
Volumes will be written on what continues to ail U.S. testing; indeed, this column has posited a few points. Policy begins with clarity: Who must be tested? How often? Why? Does everyone need a PCR (polymerase chain reaction) test every 14 days as a precursor to opening economies? How will testing be prioritized: by demographics, vocation, density of living or working conditions (e.g., schools, colleges, long-term care/ nursing home facilities, prisons)? Should Feds pay for testing and make it free to improve compliance? Should pricing be means tested or the same across all states so norms are consistently applied?
“Higher-up interference” and a likely desire to report fewer infections and faster test-reporting times led to the CDC’s August 26 reversal to not require testing for asymptomatic people even if they have “been in close contact of a person with a COVID-19 infection.”
Why do test results take so long? Could establishing higher viral load thresholds speed up reporting times and identify the infectious — not just infected — people to enable immediate contact tracing and help contain spread of the virus? Testing without contact tracing has no meaning. When will testing for antibodies scale up and how will this testing be prioritized?
POLICY ON PREVENTION AND TREATMENTS
The vaccine/drug development pipeline is impressive. One or more candidates will likely be scientifically safe and effective. Novel platforms and untested treatments, though theoretical, are exciting. But novel platforms are unproven and require thorough investigation to rule out unintended consequences. Politicians and regulators must not speculate; they must only announce quantified outcomes after scientific endpoints are reached.
While vaccines and prevention garner attention, 210 drug candidates to treat COVID-19 patients are in late-stage trials to establish efficacy and safety. Only two are currently authorized for emergency use. None have been approved. A public health emergency justifies HHS/FDA to authorize the emergency use of unapproved investigational products for treatment of laboratory-diagnosed severe COVID-19 patients. Such authorizations lack thorough reviews; but uncontestable, quantified outcomes must demonstrate benefits outweighing risks and inter-agency agreement must be a prerequisite for EUA.
20/20 hindsight will forever reflect the likely 200,000 American lives lost. IHME (Institute for Health Metrics and Evaluation) estimates that likely 310,000 will die before COVID-19 is tamed. Losing any more to politically coerced, flawed drug approval and disease reporting processes will be permanent blemishes on our nation’s ability to care for citizens. Petty politics must not prevail. American values matter. American lives matter. Public health is national security.
---
SURESH KUMAR serves on the board of Jubilant Pharmaceuticals and Medocity. Formerly, he was U.S. Assistant Secretary of Commerce and executive VP at Sanofi.