What Does Patient-Centricity Really Mean … And Why Do We Need It?
By Jeffrey S. Handen, Ph.D., director in the Life Sciences practice, Grant Thornton LLP
The challenge to recruit and retain numbers of patients for Phase 3 industry-sponsored trials is not new to the industry. In fact, 48 percent of sites miss their enrollment targets1 and 80 percent of trials are delayed due to recruitment,2 but there are new opportunities to achieve recruitment goals while also minimizing dropout.
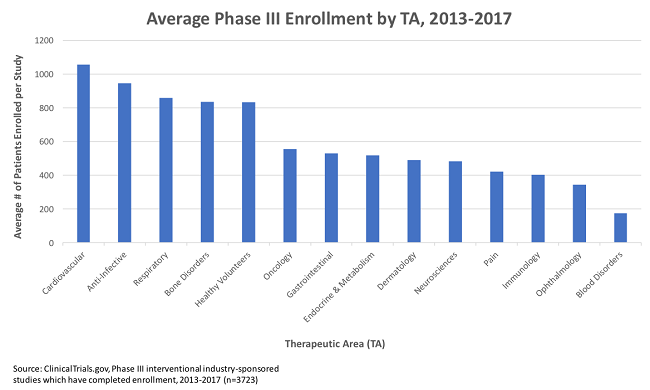
Looking at all Phase 3 industry-sponsored trials completing enrollment between 2013 and 2017 (n=3,723), there is an average enrollment of 622 subjects per trial (ClinicalTrials.gov). It is not uncommon for studies across a wide range of therapeutic areas to have thousands of subjects, and, increasingly, we are seeing mega-trials with tens of thousands of subjects. One hundred twenty-four studies in this cohort alone had enrollments above 2,000, and 28 studies had enrollments in excess of 10,000. But there are tools that now allow for better focus on patient experience and, ultimately, better trials.
Engaging The Voice Of The Patient
While Quality by Design (QbD) is increasingly being used as an effective tool in protocol development, and epidemiology and claims data informs feasibility and inclusion/exclusion criteria, very few sponsors engage potential subjects during protocol development, such as, for example, sharing nontechnical versions of the protocol outline or protocol fact sheets for feedback and input. The fact that “protocol complexity/burden” is often defined as the burden on the site or PI is evidence enough that the industry has failed to engage the potential clinical trial population.
Collecting direct patient feedback on the protocol, though ideal, is often viewed as constrained by organizational barriers, such as cost and time. We often hear “my study team doesn’t have the time to meet with patients, or “it’s a long and expensive process to engage advertising and patient recruitment vendors for every protocol. And it’s too complicated to translate a scientific protocol to laymen’s terms.” However, patient burden is a key factor in recruitment and retention, and alternate approaches exist that can quickly and efficiently address patient burden when cost and time do not justify a specific patient focus group.
Proactively gathering data from patients by leveraging disease registries and patient advocacy organizations (indication-specific) or researching populations with similar demographics to those of expected trial participants (indication-agnostic) through online surveys is one such approach. Perceptions of burden include:
- Number of required visits
- Length of visits
- Geographic distance
- Possibility of placebo
- Potential degree of invasiveness
For instance, a visit of less than 4 hours carries a severe score on some rating tools, but a visit of less than 1 hour carries a moderate score. In this way, marrying the qualitative scoring from a variety of generic trial characteristics can be used to create more standardized perceptions of burden and build algorithms to quantify a patient burden index that can be applied to all future studies.
Patient journey maps are another useful tool borrowed from the healthcare environment and applied to clinical trials to design protocols and operational processes. They can be created through a relatively small number of focused interviews with patients and/or caregivers and provide the best chance of maximizing data collection from the subject population. Developing a patient journey map starts with identifying areas of opportunity for patient identification by the sites and includes understanding of:
- how patients experience their disease
- how patients define success -- ensuring that statistically significant endpoints translate into meaningful improvements in quality of life
- real-world implications of trial design, including the frequency of patient visits, the schedule of events, ease of transportation to the site, number and invasiveness of procedures in relation to standard of care (this is the patient’s risk/benefit assessment), and what can be done to mitigate these burdens
Going Digital To Reduce Patient Burden
While the “siteless trial” may be more visionary than reality at this time, the use of electronic clinical outcomes assessments (eCOAs), wearables, and other site-remote approaches hold great promise to minimize the burden of frequent site visits by the patient while maximizing data collection.
Additional benefits include the use of today’s two-way smart technologies that allow confidential site communication to be shared directly between the site and the subject — and it’s more than just reminders, as individual subject data and lab results are also part of the exchange. To reap the benefits of engaging the subject as a true partner in clinical research, sponsors and sites will need to change the way they think about subjects — from passive patients upon which procedures are inflicted to active stakeholders who are part and parcel of the study team.
A significant challenge to incorporating these sorts of digital tools into clinical research is simply evaluating which tools to leverage. With advances in digital clinical research innovations evolving at dizzying rates, the need for organizations to assess opportunities quickly and efficiently is greater than ever. This assessment, or “sandboxing,” must be able to accommodate a myriad of potential technology solutions, provide rapid and efficient readouts, and not interfere with adherence to GCP and all such relevant regulations that ensure human subject protection and clinical trial results reliability.
Examples of digital innovations include:
- online data portals that track dosing
- smartphone apps and/or the use of voice-activated technologies such as Amazon Echo and Apple Siri that encourage patient adherence and facilitate patient-doctor communications to reduce dropout and lost-to-follow-up rates
- electronic drug labeling
- social platforms to facilitate recruitment and define the patient experience for input into protocol development
- Big Data analytics driving risk-based monitoring
- telemedicine, smartphones, and other wireless cloud-based technologies that promise the siteless trial, where endpoint data is collected without ever having to go to the doctor’s office
- synthetic trial arms
- semantic technologies to analyze unstructured data from scientific literature and social media to gain clinical insights
- leveraging EMR/EHR data for feasibility assessments and recruitment
- online PI training
Sandboxing does not necessarily require large and complex investments in clinical trial modeling and simulation (M&S) software. Utilizing a process modeling approach inspired by backward chaining and goal directed project management, a configurable financial and resourcing model can be developed to assess the impact of potential enabling technologies. Starting with an intent to positively impact time, cost, and quality, and working backward to identify critical path processes and activities (the levers that drive these results), a typical study model can be developed. Phase and TA-specific baseline model configurations can be developed using company-specific or industry benchmarking data.
Examples of Study Levers
|
End Result |
Lever |
|
Time |
|
|
Cost |
|
|
Quality |
|
Proposed solutions are assessed against which of the levers they potentially modify and where the impact can be quantified. Using a “conference-room-pilot” prior to implementation, multiple digital technologies can be compared to each other to measure their hypothetical impact. Multiple similar technologies (or different ones) from different vendors can be segmented to different “digital technology treatment arms.” With projected effects hypothesized, implementation then proceeds with metrics and other leading indicators collected during a trial period in an actual study. Following the evaluation period, generally less than three months, an interim data analysis is performed to assess the efficacy of the various proposed digital technologies and quantify the actual vs. proposed efficiencies. Additionally, a Net Promoter Score (NPS) analysis is performed to assess qualitative impact from relevant stakeholder groups. Combining the quantitative and qualitative responses allows for an “adaptive” strategy to drop the underperforming digital technology “arms” and switch the remainder of the trial to the proven digital technology.
This straightforward approach allows for constant line of sight to end-state quantifiable goals and requires relatively simple inputs once the model is developed. The inputs are defined solely as the expected levers touched by a digital technology, the projected efficiencies, a single-question NPS survey following the evaluation period, and the percent efficiencies achieved during the evaluation compared to the projections. In many cases, actual implementation itself is not needed and decision criteria can be met based on conference-room pilots.
The need to realize opportunities for digital tools in clinical research will only increase, and the myriad of vendor offerings in this space will only continue to multiply. A recent study found that 64 percent of researchers have used digital health tools in their clinical trials, and 97 percent plan to use these tools in the next five years.3
Encouraging Compliance Through Technology
While the dropout rate of clinical trials has historically hovered around 30 percent, the effects of noncompliance are, by their nature, impossible to quantify. In addition, noncompliance adds a potentially significant confounding factor. Clinical trial adherence rates have been reported to average from 43 to 78 percent among trials addressing chronic conditions.4
One solution is to leverage the power of digital. By 2020, most estimates of global smartphone penetration are above 50 percent.5 And in the developed world encompassing the traditional pharma major markets, smartphone usage is currently above 70 percent of the total population.5 Even in the so-called BRICK zone (Brazil, Russia, India, China, Korea), smartphone penetration is currently around 48 percent.5 All of this leads to the opportunity to engage subjects for enhanced compliance, not only as alarm clocks and scheduling reminders, but through communication, social media strategies, and even directly observed therapy.
In the age of social media, clinical operations teams should consider adding a new role, that of social media manager, who can communicate through the sites’ clinical research coordinators (CRCs) or study apps to keep patients engaged and active. Similar to social influencers in the online world, clinops social media managers can leverage everything from gamification to nonmedical indication-specific chats to emotional support and encouragement, and beyond, to build and maintain the study participants’ community, contributing to retention, compliance, and the patients’ experience in the clinical trial. Like all effective social influencers, the hallmarks of good patient engagement include:
- Trust
- Relatability
- Proactiveness/timeliness
- Active vs. passive engagement
- Brevity
- Positivity
- Some degree of knowledge (but not necessarily expertise)
This can be operationalized via the CRC’s direct communications to patients or a patient-focused study-specific app or website, and since it does not involve the collection or transmission of any clinical data, no validation or regulatory compliance is required. While perhaps not practical for small studies, this investment can reap huge rewards in larger and longer-term studies.
The phrase “customer centricity” continues to be a term that confounds and is defined very differently depending on who you are speaking with. We’d love to hear what customer centricity means to you and how your organization is adapting. We hope you’ll take a moment to leave a comment below.
References:
- Tufts CSDD Impact Report, 2013, 15(1), K. Kaitin, ed.
- Drugdevelopment-technology.com, Clinical Trial Delays: America’s Patient Recruitment Dilemma, 2012, D. Garrum,
- Validic, 2016, Insights on Digital Health Technology Survey 2016
- N Engl J Med 2005;353:487-97, Adherence to Medication, L. Osterberg and T. Blaschke
- Zenith, 2017, Mobile Advertising Forecasts 2017; Statista, 2017, Smartphone user penetration as percentage of total global population from 2014 to 2021; eMarketer, 2017, 2.4BN Smartphone Users In 2017. D. Murphy
About The Author:
 Jeffrey S. Handen, Ph.D., is a senior management consultant at TayganPoint Consulting Group and a leader in the firm's Life Sciences R&D Practice. He has published in multiple peer-reviewed and business journals, presented at numerous industry conferences and scientific meetings as an invited speaker, served as past editor in chief of the Industrialization of Drug Discovery compendium, and currently serves as the editor in chief of Re-inventing Clinical Development. Handen has over 20 years’ experience in pharmaceutical and biotechnology R&D, process re-engineering, and systems and process implementation. He is responsible for overseeing business process improvement and solution architecting for optimizing both clinical development and discovery.
Jeffrey S. Handen, Ph.D., is a senior management consultant at TayganPoint Consulting Group and a leader in the firm's Life Sciences R&D Practice. He has published in multiple peer-reviewed and business journals, presented at numerous industry conferences and scientific meetings as an invited speaker, served as past editor in chief of the Industrialization of Drug Discovery compendium, and currently serves as the editor in chief of Re-inventing Clinical Development. Handen has over 20 years’ experience in pharmaceutical and biotechnology R&D, process re-engineering, and systems and process implementation. He is responsible for overseeing business process improvement and solution architecting for optimizing both clinical development and discovery.
