What's Next For Single-Use Technology In Bioprocessing

By Luke Heaven, Sartorius Stedim Biotech
This year marks the 21st anniversary of single-use bags in bioprocessing. Since the first products were introduced to the market in 1995, single-use systems (SUS) have gone from being a niche technology to replacing plastic carboys to becoming a mainstay of industrial-level bioprocessing (and, to a lesser extent, industrial-level pharmaceutical processing). Based on our analysis of market research data, we estimate that the current global market for single-use products is well over $600 million.
The advantages of single-use are well-known — faster, smaller, greener, cheaper, and safer — and increasing numbers of biomanufacturers have sought to reap these benefits, especially as patent expiration and increasingly globalized manufacturing continues to change the dynamics of biomanufacturing.
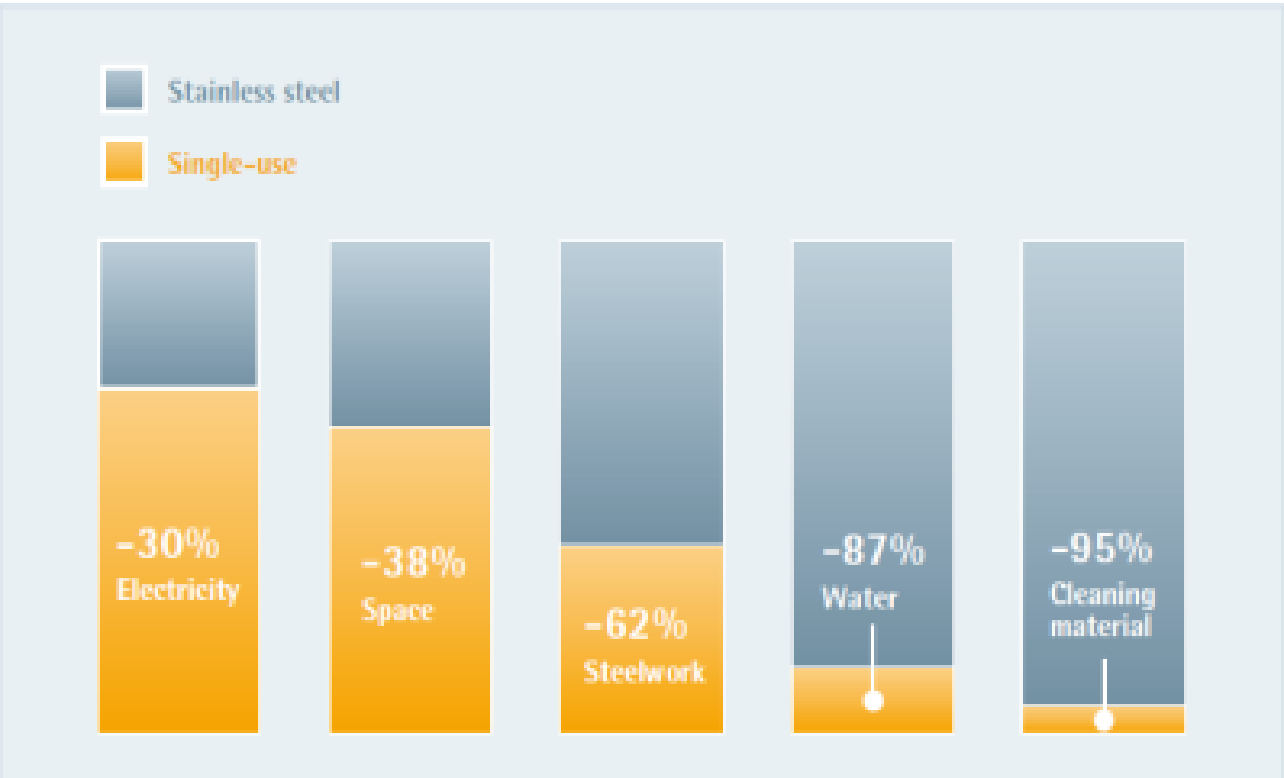
Figure 1: Environmental comparison of single-use and stainless steel 1
The rapid growth and implementation of SUS has driven increases in volumetric scale capability from simple low-volume storage bags to progressively larger volumes, with 3000-liter bags generally accepted as the upper limit with respect to safe handling. Innovations such as sophisticated mixing systems, aseptic tube connection devices, sensor technology advancements and automation have enabled the evolution of single-use systems into fully fledged unit operations, including the latest generation of single-use bioreactors. Other recent advances such as capsule filtration, aseptic connection devices, and tube welders for thermoplastic tubing have been adopted in such a widespread manner that they have become almost standard practice. Mixing technology, in particular, has seen substantial growth as low-shear mixing technologies have enabled single-use solutions to be used in downstream processing as well as media and buffer preparation.
The integration of all these elements of SUS has made the technology more accessible to end users. This is exemplified by the availability of vendor-supplied impeller mixing bags with pre-attached filter lines, aseptic connectors, and in-line disposable pH and conductivity sensors, which can be used with thermo-regulated totes fitted with integrated load cells.

Figure 2: Typical bioprocess production platform utilizing SUS
SUS has drastically changed the way many drug manufacturers serve their patients. This is realized not only in time and cost savings from eliminating the need for sterilize-in-place (SIP) and clean-in-place (CIP) cycles, but also in the additional flexibility and capacity utilization gained from having a disposable process train that can be replaced, modified, or removed with minimal cross-contamination risks. As a result, drug manufacturers can quickly adapt their global manufacturing resources to meet changes in demand, enabling them to both serve existing patients and open up new markets — while simultaneously managing the impact and opportunities of generic and biosimilar production resulting from patent expiration.
If the single-use revolution is indeed here to stay, the question is, “What’s next for the technology?” The answer could simply be maturation.
The application of single-use technology in increasingly critical process steps, such as final bulk drug product storage and formulation and filling operations, means that end users’ challenges and needs are shifting more from cost evaluation to implementation. Surveys conducted by Aspen Brook (6th Annual Survey of the Single Use Bioprocessing Market) and BioPlan Associates (12th Annual Report and Survey of Biopharmaceutical Manufacturing Capacity and Production) demonstrate that the main issues related to SUS concern easing its implementation. End users cite quality assurance, supply chain reliability, change control, raw material transparency, and integrity testing as necessary areas of evolution for SUS.
To address these concerns, single-use suppliers are using a mix of material science, end user support and training, validation, documentation and standardization of materials, design, and connection methodologies (e.g., the use of vendor-independent connectors and thermoplastic tubing) to make the implementation of SUS as smooth as possible. End users and collaborative initiatives such as the Bio-Process Systems Alliance (BPSA) and BioPhorum Operations Group (BPOG) are working to set standards to improve the use and acceptance of SUS.
Even engineering firms, once the biggest detractors of SUS, are embracing the technology and actively promoting standardization projects. Dave Wolton of the PM Group is spearheading a cross-functional collaboration for standardization between vendors (Standardized Disposable Design Initiative). “Over the last three years, I have had a great response from both suppliers and end users regarding our standard disposable design initiative” Wolton says. “We launched end user access to the 200+ designs in 2015, and from day one we plan for these designs to be available from up to six leading suppliers.”
An extensive process of raw material control is also a key area where SUS vendors are focusing their efforts. The recent reported cell growth issues in some single-use bags linked to the presence of bis 2,4-di-tert-butylphenyl phosphate (bDtBPP) — as a degradation product from the antoxidants used in many film formulations — has highlighted the need to both optimize and control all raw materials and additives and to apply a QbD approach to the entire production process, from resin to bag.
Detailed control of materials all the way back to the resin is also serving to facilitate and assure repeatability of material characterization processes. Extractable analysis remains a key focus for end users and regulators alike. Therefore, control and optimization of raw materials, including the identification and strict specification of all additives used in the film extrusion process, facilitates easier toxicological risk assessment.
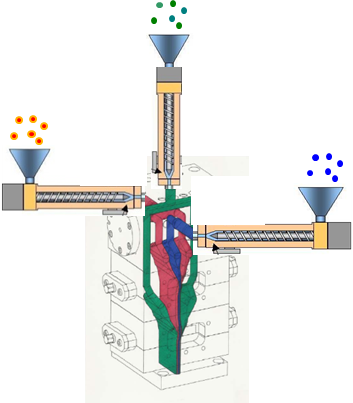
Figure 3: A typical film formulation process for films used in SUS
Perhaps the most important learning for SUS vendors, however, was the need to offer enhanced assurance of supply of single-use products. As the implementation of SUS effectively constitutes an outsourcing of the production pathway to single-use vendors, the implementation of SUS at an industrial scale means that end users demand assurance of rigorous change control practices, detailed specifications, and design space establishment for SUS, as well as continuity of supply. Redundant capacities, strong contracts, and security stocks all serve to add the level of supply chain assurance required.
In terms of technology development, the most interesting progress will likely be in the field of process analytical technologies and automation. Fully automated solutions including process monitoring and recording are in high demand and the use of in-line disposable sensors such as glass pH electrodes and conductivity sensors are driving the new “smart” generation of single-use systems.
The industry can also expect significant advancements in technology and strategies concerning closure container integrity. Sophisticated solutions in the field of SUS integrity testing will become more prevalent, with coherent strategies for mitigating the risk of both macro and micro leaks closely aligned with capabilities and potential failure modes.
As SUS implementation continues, we will likely also see a clear differentiation and segmentation of where in the process it is used. Differing commercial and regulatory requirements for buffers, bulk drug substance, and bulk drug product will drive an evolution of pre-defined solutions for different process steps. Such solutions will more closely align designs, technology development, and QC testing with the needs of different process steps and associated regulatory expectations.
In summary, single-use technology has followed a path of maturation not to dissimilar from our own. Since its inception, it has learned, it has evolved, and as it now comes of age, it is taking on new responsibilities associated with its phase of development. This is something to which we all can relate.
References:
- Sinclair, A.; Leveen, et al.; The Environmental Impact of Disposable Technologies, The Biopharm International Guide, November 2008; Base of the analysis: Typical mAb process at 3 X 2000 L scale
About the Author
 Luke Heaven is director of marketing for Europe, Asia, NEMEA, and Cuba for fluid management technologies at Sartorius.
Luke Heaven is director of marketing for Europe, Asia, NEMEA, and Cuba for fluid management technologies at Sartorius.
