What To Know About Post-Grant Review And The Biotech Industry
By Brian C. Kwok, Nicholas V. Martini, and Nicole Johnson
Patent litigation can often be a costly, lengthy, and resource-intensive endeavor. Recognizing the frequency, duration, and potentially debilitating effects of patent litigation, Congress passed the America Invents Act (AIA) in 2011.
The AIA made several significant changes to United States patent law, including the establishment of post-grant proceedings before the Patent Trials and Appeals Board (PTAB) where patents have the potential of being invalidated in a much more efficient and expedient manner. In the five years since the AIA was enacted, two types of post-grant proceedings, Inter Partes Review (IPR) and Post-Grant Review (PGR), have become increasingly popular tools for biotechnology companies to resolve patent disputes.
A COMPARISON OF LITIGATION, IPR, AND PGR
To use post-grant proceedings before the PTAB effectively, it is important to observe how patent invalidity challenges in district court compare to post-grant proceedings (i.e., IPRs and PGRs) before the PTAB. Some sophisticated strategies integrate both district-court proceedings and post-grant proceedings before the PTAB to effectively stage a multifront attack during patent disputes. For example, some strategies may employ a combination of both district court litigation and an IPR proceeding before the PTAB to increase the likelihood of a favorable outcome and oftentimes increase the motivation for parties to settle sooner or position a case for success should litigation ensue.
CURRENT TRENDS FOR BIOTECHNOLOGY PGRs
A total of 10 PGRs have been filed in the biotechnology space. From 2015 to 2016 the number of PGRs filed in this space has more than tripled from two to seven petitions filed within the year, which is significant because the number of patents eligible for PGR (those with a priority date on or after March 16, 2013) are just starting to be issued in greater numbers. Some commentators (including judges) predict that PGR will continue to increase in popularity as the number of eligible patents continues to grow. Getting ahead of this trend and considering PGR as part of an overall patent strategy would behoove any company faced with patent disputes.
Currently, about one-fifth of all PGRs filed have been in the biotechnology space (1,600), making that the most popular field for PGR filings.
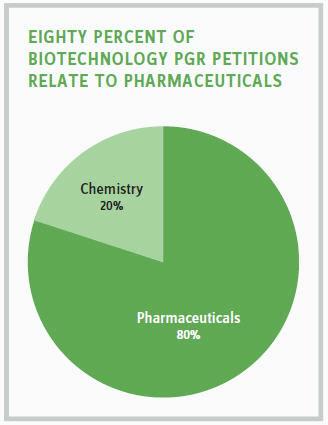

Of the 10 petitions relating to biotechnology that have been filed, three have been denied, two have been granted, one is awaiting institution decision, and one final decision was issued. The remaining three PGRs were terminated by the parties — two before institution and one after institution. The PGR petitions that have been filed in the biotechnology space have been related to either chemistry or pharmaceuticals, with the majority involving pharmaceutical-related technologies.
TYPES OF CHALLENGES IN BIOTECHNOLOGY PGRs
Perhaps not surprisingly, challenges to the written description of a patent, including enablement and indefiniteness, have been the most popular invalidity challenges in PGR petitions in biotechnology. When patents claim large genera of chemical or pharmaceutical compounds, questions can arise as to whether the patent adequately permits a skilled artisan to make and use all of the compounds claimed. These types of challenges are particularly suited to PGR challenges, especially when stakeholders are more interested in invalidating a patent based on written description and enablement challenges than traditional prior art challenges.
INSIGHTS FROM PTABs TO BIOTECHNOLOGY
Altaire Pharamceuticals, Inc., v. Paragon BioTeck, Inc. was a PGR filed on May 11, 2015, instituted on November 16, 2015 and decided November 14, 2016. In this decision, the PTAB held that the petitioner did not meet its burden of proof to demonstrate that the challenged claims were unpatentable. Therefore, the PTAB ruled in favor of the patent owner.
In this case, the petition challenged U.S. Patent 8,859,623, claiming that it was unpatentable as obvious over the petitioner’s product. The case turned on whether the evidence provided by the petitioner was sufficient for the PTAB to find unpatentability. The issue with the petition was that the tests and data it submitted did not meet the requirements of 37 C.F.R. § 42.65 (the petition failed to explain how the test was performed and how the data was generated) and other data used by the petitioner was unreliable. Thus, the problem for the petitioner was not that the PTAB did not find its PGR petition flawed; it was that the underlying evidence supporting its arguments did not comply with the relevant procedural rules.
With more patents being eligible for PGR, we will likely see filings in this space continue to increase. If the trend in biotechnology IPRs is any indication of what is to come with PGRs, stakeholders need to be prepared to defend themselves if they are faced with a PGR or, alternatively to have considered PGR as part of their current patent strategy.

The opinions expressed are those of the authors and do not necessarily reflect the views of the firm or its clients, or any of its or their respective affiliates. This article is for general information purposes and is not intended to be, and should not be taken as, legal advice.
