Bloated COVID Relief Package Misses Mark
By John McManus, president and founder, The McManus Group
 Dear reader, it has been my pleasure to write a monthly column for Life Science Leader for the past seven years, and this is my final column. Hopefully, it has provided insight into the “sausage-making” process of Washington that produces public policy impacting the life sciences sector.
Dear reader, it has been my pleasure to write a monthly column for Life Science Leader for the past seven years, and this is my final column. Hopefully, it has provided insight into the “sausage-making” process of Washington that produces public policy impacting the life sciences sector.
One thing you may have concluded from my observations is that hyperbole and populist rhetoric — on both sides of the aisle — too often outweigh data and objective analysis. But that’s the unfortunate current nature of politics. Inflaming and channeling public outrage for personal or partisan political advancement can result in serious, untoward collateral damage that adversely impacts the very patients that politicians purport to be assisting.
My old boss, Ways and Means Chairman Bill Thomas, used to say, “Politics is the process of deciding who gets what, when, and how.”
Pharmaceutical Manufacturers’ Tough Political Spot
With a limited footprint in a handful of ZIP codes, the pharmaceutical industry is politically vulnerable. The convoluted pricing and reimbursement channels that have developed over the years appear to penalize patients with escalating out-of-pocket costs at the pharmacy counter even though net prices have remained stable due to substantial and increasing rebates to pharmacy benefit managers (PBMs) from manufacturers. Seniors, probably the most powerful voting bloc in the country, are particularly reliant on numerous medications to keep them healthy and are disproportionately affected by such cost increases.
Politicians in both parties capitalize on the confluence of these factors to vilify the pharmaceutical industry and suggest simplistic, populist solutions. It is a remarkable testament to the industry that international reference-pricing schemes or outright government price controls have not yet been implemented in Medicare.
Democratic control of Congress — albeit with razor-thin margins (five votes in the House and Vice President Harris breaking a 50-50 tie in the Senate) — provides an opportunity to jam through legislation this year that could fundamentally transform pharmaceutical reimbursement in Medicare, Medicaid, and even commercial plans.
Yet the industry’s remarkable achievement of delivering multiple extremely effective vaccines to combat the most significant pandemic in a century may have bought it some goodwill. While policymakers appropriately fret over the proper and efficient deployment of the vaccine, it’s important to not lose sight of the record speed and effectiveness of the COVID vaccines.
The World Economic Forum notes that vaccine development typically takes 10 years from discovery to market approval. In contrast, three drug companies researched, developed, and worked collaboratively with regulators to bring their vaccines to market in a matter of months, with three other companies on the brink of successful vaccine development. Moreover, both Pfizer’s and Moderna’s vaccines are more than 90% effective, in contrast to the flu vaccine, which is typically only 40% effective.
While supply has not initially met demand, manufacturers are ramping up production, and Pfizer is on track to produce 300 million doses of its two-shot vaccine by the end of June. Novavax and AstraZeneca expect approval for their vaccines in the next few months. With this increased supply, it is not hard to imagine that most Americans should be vaccinated by the end of Q2.
$1.9 Trillion COVID Package Misses Mark
The end of the pandemic is in sight, yet Congress is now about to enact a staggering $1.9 trillion spending bill with less than 9% targeted to combat COVID through vaccinations, testing, and tracking of the virus and half spent in 2022 and beyond.
Congress has already enacted four bipartisan COVID relief bills in 2020, the most recent in December with $1 trillion still unspent. Despite President Biden’s call for bipartisanship, this bill is being run under “budget reconciliation” through a totally partisan process. Democrats accepted only two of 229 amendments offered in the nine committees that marked up the bill.
Nearly one-third of the total Coronavirus Relief Fund remains unspent and is intended to assist state and local governments to cover unanticipated expenditures due to projected revenue shortfalls. However, those grim projections never materialized. The Tax Foundation reports that state and local government tax collections were down just 0.7% for the first nine months of 2020 compared to the same time period in 2019. Yet the bill devotes $510 billion to state and local governments under a formula that provides more funds to states with higher unemployment, those states that are more likely to have shut down their economies.
Of the $128 billion devoted to reopening schools, just 5% is slated for this year, according to the Congressional Budget Office. Teachers unions continue to demand they will not return to in-person learning until they are vaccinated and ventilation systems are retrofitted. Why must ventilation systems be retrofitted if people are vaccinated, and how long will that take? Many local school systems are already stating that they cannot return to in-person learning this fall!
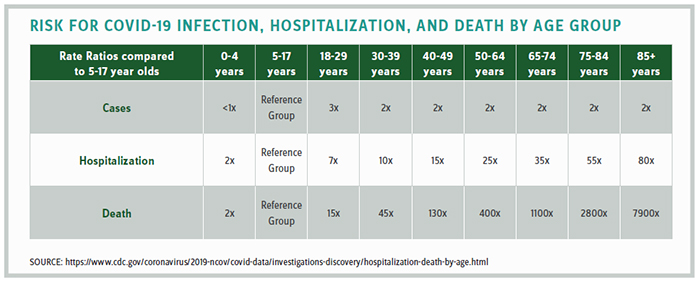
The science does not support the notion that school-age children are at risk, as the CDC statistics in the chart above clearly demonstrate. Yet the negative impact to children of not returning to school is significant. In February, the president of the Virginia Academy of Pediatricians testified in the legislature that their doctors report a 97% increase in anxiety, 95% increase in depression, 87% increase in behavioral problems, and an 84% increase in obesity.
The legislation appears to anticipate more and extended parental time at home to monitor the virtual learning of their kids by providing $1,400 paid leave a week for up to 15 weeks to federal employees.
Why not just open the schools across the country as many communities across the south have successfully done from the get-go?
Concluding Thoughts
I thank the editors of Life Science Leader for providing me the platform to provide substantive, data-driven reporting and analysis of legislative and regulatory developments that impact the pharmaceutical and broader healthcare sectors. They’ve given me leeway to write about topics of my choosing, including the enactment and implementation of the Affordable Care Act, healthcare provider consolidation, international reference pricing schemes, pharmaceutical reimbursement, tax policy, and a slew of other policies too numerous to recount here. All of my columns are archived on our website: www.mcmanusgrp.com and I welcome any feedback on my work over the years — negative and positive — jmcmanus@mcmanusgrp.com.
John McManus is president and founder of The McManus Group, a consulting firm specializing in strategic policy and political counsel and advocacy for healthcare clients with issues before Congress and the administration. Prior to founding his firm, McManus served Chairman Bill Thomas as the staff director of the Ways and Means Health Subcommittee, where he led the policy development, negotiations, and drafting of the Medicare Prescription Drug, Improvement and Modernization Act of 2003. Before working for Chairman Thomas, McManus worked for Eli Lilly & Company as a senior associate and for the Maryland House of Delegates as a research analyst. He earned his Master of Public Policy from Duke University and Bachelor of Arts from Washington and Lee University.
