Congress Tackles The Opioid Crisis
By John McManus, The McManus Group
 After passing a gargantuan appropriations bill that balloons the deficit by hundreds of billions and funds the government through October, the conventional wisdom in Washington is that Congress is done legislating for the rest of the year, save one major issue: addressing opioids.
After passing a gargantuan appropriations bill that balloons the deficit by hundreds of billions and funds the government through October, the conventional wisdom in Washington is that Congress is done legislating for the rest of the year, save one major issue: addressing opioids.
Opioids are narcotic pain relievers and are available only by prescription. When used as directed, they are an important component of fighting acute (e.g., postsurgery or cancer) or chronic pain. But they also can provide a highly addictive physiological high, particularly when manipulated for injection and snorting. When used in high quantity, they can lead to respiratory failure and death.
The breadth and impact of the opioid crisis has scourged vast portions of the country — affecting people of all demographic groups. According to Pew Charitable Trust in 2016, 64,000 died from overdoses — triple that of 1999, surpassing automobile crashes and homicides as the leading cause of death for Americans under age 50. Opioids are the cause of the first decrease in life expectancy in 25 years. More than 1 million otherwise able-bodied people have dropped out of the workforce, resulting in the loss of over $700 billion in economic output.
The CDC found that drug overdose deaths fell in 14 states, yet it’s not clear whether that represents a sustainable trend. Caleb Alexander, codirector of Johns Hopkins University’s Center for Drug Safety and Effectiveness, commented, “If we’re truly at a plateau or inflection point, it would be the best news of the year. But we’re still seeing rates of overdoses that are leaps and bounds higher than what we were seeing a decade ago and far beyond any other country in the world.”
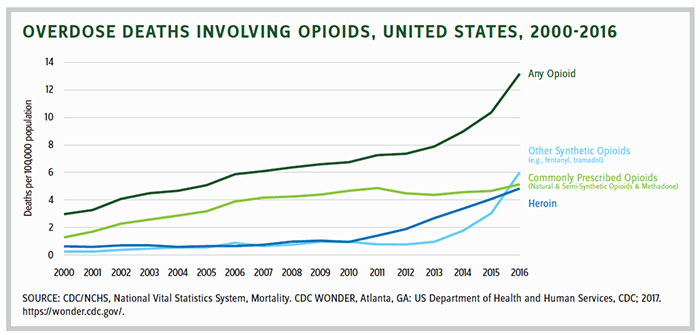
Congress is now mulling scores of bills that would address different aspects of the crisis. Four congressional committees have held exhaustive hearings examining nearly 100 bills on the issue. Later this month, they will begin marking up legislation that builds on the Comprehensive Addiction and Recovery Act (CARA) of 2016 and distributes the $6 billion that was provided in the Bipartisan Budget Act of 2018 for opioid abuse and mental health treatment.
The goal is to report out legislation from the committees before Memorial Day and send a comprehensive package to the president in June.
The Senate HELP Committee held seven hearings since last October and Chairman Alexander (R-TN) announced that it will be marking up an omnibus bill to address multiple parts of the crisis, including increased access to addiction-recovery treatment, stronger enforcement measures, and limiting the amount of opioids being prescribed.
Senator Portman (R-OH), the author of CARA and a leader in the opioid fight, has introduced CARA 2.0, which would:
- provide a three-day limit on opioids for acute pain
- authorize $300 million annually (up from $12 million authorization in CARA) to make naloxone available and train first responders
- provide $300 million to expand evidence-based activities related to treatment for substance use disorders, including the availability of Medication Assistance Treatment (MAT)
- require physicians and pharmacists to use their state prescription-drug monitoring program
- increase civil and criminal penalties for opioid manufacturers that fail to report suspicious orders for opioids or fail to maintain effective controls against diversion of opioids.
Energy & Commerce Committee Package
The House Energy and Commerce Committee has examined more than 60 opioid-related bills over three hearings, including 34 bills in the last hearing alone. Among other things, the bills would:
- create Medicare Part B incentives to develop nonopioid drugs for postsurgical pain and a temporary pass-through payment to reimburse doctors at higher rates for prescribing these drugs
- require state Medicaid programs to integrate drug-monitoring programs into providers’ and pharmacists’ clinical workflows
- repeal the medical institutions for mental-disease exclusion for a five-year period to allow Medicaid to pay for inpatient recovery
- expand opioid-disorder treatment options through telehealth under Medicare Part B
- require Medicare PDP to establish drug-management programs for at-risk beneficiaries
- study Medicare beneficiary access to abuse-deterrent formulations under Medicare Part D.
Energy & Commerce Ranking Member Frank Pallone commented, “I am concerned that the sheer quantity of bills before the committee today and the chairman’s extremely ambitious time frame will not leave us much time to get these policies right. … At times, to me, this process feels more like an opioids media blitz than a thoughtful discussion about our national public health crisis.”
Nonetheless, the political will to act on the issue is intense; the crisis dominates town hall discussions in Democratic and Republican districts alike. Unlike most health issues, addressing the opioid crisis has no ideological or partisan divides. The real challenge, as illustrated by the plethora of legislation, is whether real solutions are at hand or is Congress simply trying to pursue a shotgun approach of a myriad of ideas.
One idea, advanced by the FDA, is the approval and suggested eventual transition of the opioid market to abuse-deterrent formulations (ADFs). These products contain chemical properties which make it extremely difficult to crush or dissolve and manipulate them for snorting and injection. Such misuse of opioids increases the maximum concentration of the opioid in the brain in a rapid manner. AD opioids can help prevent the cascade of misuse and abuse of opioids. Moreover, they may limit diversion to individuals who were not prescribed the opioids but have access to a legitimate prescription.
Although 10 ADFs have been approved in recent years, ADFs have not been widely adopted in the market, in part because plans have limited access to these branded and more expensive products in the highly genericized market. Five states have passed laws to allow greater patient access to these products, but data is not yet available on the impact of those laws on utilization of ADFs.
According to the CDC, 40 percent of opioid deaths are from Rx products; curtailing supply of abuse-prone opioids can save lives. Yes, some people will move to heroin if they are unable to inject the prescription drug. Some will anyway, but many lives can be saved. This is a war against an addiction that’s all-controlling — anything to stave off the scourge is helpful. If we can’t get access to AD opioids right, hopes of ROI for nonaddictive
products are shattered.
John McManus is president and founder of The McManus Group, a consulting firm specializing in strategic policy and political counsel and advocacy for healthcare clients with issues before Congress and the administration. Prior to founding his firm, McManus served Chairman Bill Thomas as the staff director of the Ways and Means Health Subcommittee, where he led the policy development, negotiations, and drafting of the Medicare Prescription Drug, Improvement and Modernization Act of 2003. Before working for Chairman Thomas, McManus worked for Eli Lilly & Company as a senior associate and for the Maryland House of Delegates as a research analyst. He earned his Master of Public Policy from Duke University and Bachelor of Arts from Washington and Lee University.
