-
Galien Forum: CEOs Talk FDA, China, Funding Sources11/25/2025
Part two of a two-part series covering FDA Commissioner Makary’s comments at the Galien Forum on October 30, plus coverage of a CEO roundtable on FDA, China, and funding.
-
Makary Talks Faster Drug Reviews, 'Continuous Trials,' DTC Ads10/31/2025
Part one of a two-part series on FDA Commissioner Martin Makary, M.D.'s comments at the Gailen Forum in New York City on October 30, 2025.
-
Reading The FDA Tea Leaves On Psychedelics10/17/2025
MAPS founder Rick Doblin accused the FDA of changing the goal posts after Lykos's MDMA Complete Response Letter was released, and offered insights for other psychedelic developers.
-
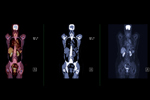
Partnering To Commercialize Radiopharmaceuticals10/9/2025
For small and midsize radiopharmaceutical developers, an experienced commercial partner can make or break the success of new products.
-
From Scientific Discovery To Next Generation Treatments For Obesity7/31/2025
Twenty years ago, Roger Cone, Ph.D. made a key discovery in obesity science which could help Courage Therapeutics deliver products that improve upon currently available treatments for obesity.
-
Epic Bio's Big Swing In Epigenetic Editing7/11/2025
Amber Salzman, Ph.D., CEO at Epicrispr Biotechnologies, joined the Business of Biotech live in Boston to talk about epigenetic editing, working with the FDA, using AI for clinical endpoints, and more.
-
BIO2025: Sanofi R&D, FDA's Martin Makary, Paying For New Therapies6/20/2025
BIO was as busy as ever in Boston this year. With more to come soon, here are a few initial thoughts on three events I attended.
-
Companies To Watch: Vivani Medical5/30/2025
Vivani Medical is developing GLP-1 subdermal implants that aim to provide a consistent therapeutic dose for six months, and possibly one full year. The company's lead program is an exenatide implant.
-
Promoting A Newly Approved Indication Against Established Competitors5/14/2025
In a follow up to his recent appearance on the Business of Biotech, Tolga Tanguler explains how Alnylam is positioning Amvuttra's new ATTR-CM indication approval against competitors in the category.