Articles By Dan Schell, Editorial Director of Life Science Leader

-
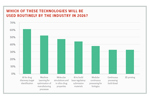
AI Predictions From The CPHI Annual Report11/1/2023
The CPhI Annual Report 2023 that was released last month includes insights from 250 global pharma companies and predicts that “within 10 years, over 50% of approved drugs will involve AI in their development and/or manufacturing.”
-
From No Experience To A New Career In Biopharma11/1/2022
Firsthand accounts from participants in MassBioEd’s new Life Science Apprenticeship Program.
-
What's It Like Being A Termeer Fellow?4/1/2022
We reached out to four previous Termeer Network Fellows to find out how the program helped them and what they learned. Participants included Sun Altbach, founder and former CEO and president of PIC Therapeutics; Ana Maiques, CEO, Neuroelectrics; Stan Wang, founder & CEO, Thymmune Therapeutuics; and Natalie Yivgi-Ohana, CEO, Minovia Therapeutics.
-
A Cover Feature Four Years In The Making3/1/2022
I’m not sure what prompted the response from Rachel Haurwitz. It was, after all, a simple “thank you” that I had sent her via LinkedIn back in November 2017 for contributing some comments to our 2018 outlook issue.
-
Is Biopharma At An Inflection Point Regarding Digital Transformation?3/1/2022
Released in late 2021, Deloitte’s, “Gain An Edge With Leapfrog Digital Innovation” report talks about how the biopharma industry is at an inflection point when it comes to digital transformation.
-
Can Caribou Biosciences Top 2021?3/1/2022
In 2021, much of Rachel Haurwitz’ hard-earned on-the-job training over the past decade culminated in a series of significant milestones that will undoubtably help position Caribou Biosciences for future growth and success.
-
Thanks To COVID, Your Culture May Need An Overhaul1/4/2022
This biotech fortified its culture during the pandemic by creating an employee support group, a new performance-management program, a woman-focused initiative, and programs that give days off for volunteering and provide $1200/year for personal and family development goals.
-
Becoming A Clinical-Stage Biotech9/1/2021
A Q&A with Laura Indolfi, CEO and cofounder of PanTher Therapeutics, which recently closed its Series A funding round and is preparing for its first clinical trials.
-
A Q&A With A Biotech Founder/Entrepreneur8/2/2021
Since cofounding Kymera Therapeutics, Nello Mainolfi, Ph.D., has served as CSO, CTO, and now is president and CEO. He helped Kymera bring the first ever targeted protein degrader to the clinic, go public in 2020, and land significant partnerships with the likes of GSK, Vertex, and Sanofi.
-
Life Sciences CEOs Assess Upcoming Business Risk6/1/2021
We take a closer look at the data related to organizational growth risks included in a recent KPMG “Global CEO Outlook” survey.
-
CEO Q&A: Dr. Aoife Brennan5/3/2021
Dr. Aoife Brennan, president and CEO of Synlogic, discusses how the pandemic has affected her company’s operations and clinical trials.
-
A Q&A With A COVID-Era New CEO5/3/2021
David Horn Solomon, Ph.D., took on the role of CEO of Pharnext in April 2020, at the beginning of the pandemic and just after the company had seen it’s Phase 3 trial data rejected by the FDA.
-
A Q&A With Bernie Zeiher Of Astellas4/1/2021
Bernie Zeiher Of Astellas took on the dual role of CMO & President of Development in April 2018. Here he discusses how he adjusted to that job and some of the best practices he’s learned along the way.
-
CEO Q&A: Mary Szela2/1/2021
Mary Szela spent 25 years in Big Pharma and the last 7 at three small biotechs. The CEO Of TriSalus Life Sciences discusses how she adapted during these career changes and some of the difficulties she encountered along the way.