CohBar: Producing Mitochondria Medicine
By Wayne Koberstein, Executive Editor, Life Science Leader
Follow Me On Twitter @WayneKoberstein
The Enterprisers: Life Science Leadership In Action

Why shouldn’t our mitochondria want us to live long, prospering in good health? Why shouldn’t they — as symbiotic microbes turned cellular organelles with their own mini-genomes — carry genes that help ensure our healthful survival? After all, they ride around inside our cells, living on what we feed them, as they transform those elementals into the chemical fuel that powers us. In a variety of ways, they reach out and help keep our bodies whole and healthy as well. Many companies have sprung up around the idea of making medicine based on mitochondria’s role in health and disease, and one of them, CohBar, has chosen a unique way that avoids the common challenge of invading the cell’s interior to achieve therapeutic effects.
Mitochondria also play a big role for many of us in our imaginations. Children and adults who have read and related to the classic fantasy, A Wrinkle in Time, can envision the organelles as a forest of tiny, self-aware creatures who can be summoned to action against an invading evil. “Everybody’s got a parallel to Wrinkle,” says Albion Fitzgerald, chairman of CohBar. The book had just enough science, based on the current knowledge when published in 1963, to teach and touch off interest in the inner workings of the living cell. For many, it was the first step in a lifetime quest to look ever deeper inside the biology of cellular life.
For others like Fitzgerald, the book may have simply accelerated an interest in the principles that govern organized systems, including the computer-based applications and business startups that preoccupied most of his career. An interesting part of CohBar’s singular story is how it attracted the IT architect and entrepreneur onto a new professional path as an involved board chairman in a biopharma enterprise.
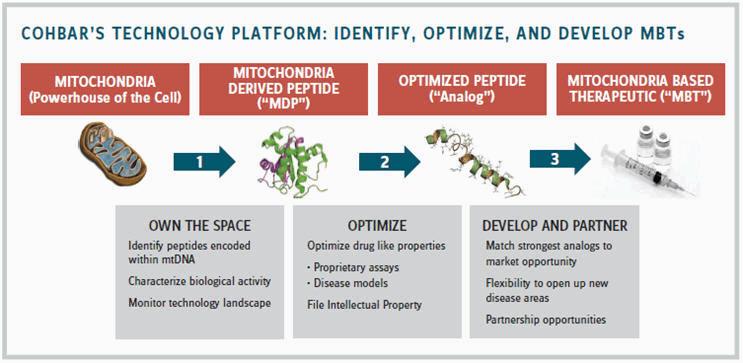
Fitzgerald and CohBar’s CEO, Simon Allen, joined me for a conversation at the October 2016 BIO Investor Forum to offer the company’s novel take on the mitochondria. CohBar creates analogs of certain peptides produced by mitochondrial genes to generate health-maintaining proteins the natural peptides normally express. It systematically discovers, analyzes, optimizes, patents, and produces selected mitochondrial-derived peptides (MDPs) as therapeutic agents. Founded in 2007, but with its origins going back 20 years, the company recently emerged from a long period of research and refinement to enter preclinical drug development, building on the considerable body of science its founders created.
 As they walk into the room, Allen has just made the company presentation at the Forum, and Fitzgerald temporarily takes the lead in explaining the company’s starting premise, offering a layman’s version of its scientific foundation. “Our founders, Dr. Pinchas Cohen and Dr. Nir Barzilai, discovered a whole class of peptides coded inside the mitochondria that affect metabolic regulation and protection,” he says. “They had just discovered the new genes in the sequence of the genome and were trying to figure out the function and beneficial effects of each gene.”
As they walk into the room, Allen has just made the company presentation at the Forum, and Fitzgerald temporarily takes the lead in explaining the company’s starting premise, offering a layman’s version of its scientific foundation. “Our founders, Dr. Pinchas Cohen and Dr. Nir Barzilai, discovered a whole class of peptides coded inside the mitochondria that affect metabolic regulation and protection,” he says. “They had just discovered the new genes in the sequence of the genome and were trying to figure out the function and beneficial effects of each gene.”
The genome sequencing and discovery of a new class of peptides dramatically increased the long-believed number of known mitochondrial genes, from 37 to over 80, and greatly expanded the number of possible peptides they encode. The founders’ next thought, prompted by some serendipitous clinical data, was to identify which of the MDPs might represent the most useful proteins for therapeutic purposes in conditions related to metabolic regulation and protection.
Subsequently, CohBar made a further, enterprising leap ahead: Synthesize analogs of the MDPs for development as mitochondria-based therapeutics (MBTs). To date, research studies have identified potential MBT candidates for treating Type 2 diabetes (T2D), Alzheimer’s disease, obesity, fatty liver, and certain cancers. CohBar’s lead candidates, coded CB4209 and CB4211, are analogs of the MOTS-c (mitochondrial open reading frame of the 12S rRNA-c) peptide discovered by Cohen and his USC colleagues that show strong therapeutic potential for obesity, T2D, and NASH (nonalcoholic steatohepatitis, associated with fatty liver) in the company’s preclinical models. Clinical testing remains more than a year away; CohBar plans to launch a Phase-1a trial in early 2018.
That means the initial human trial will start more than 15 years after the first MDP, humanin, was discovered and the CohBar founders began to investigate its effects. In 1998, Barzilai founded (and currently oversees) an ongoing study to identify genes that promote long life called the Longevity Genes Project. Among the findings from this study, using a novel assay developed by Cohen, was that centenarians and their offspring exhibit much higher levels of humanin in their blood than individuals with average life expectancy; the same subjects also had extraordinarily disease-free lives. Suspecting the two trends were related, the CohBar founders studied how humanin acts to promote lifespan and healthspan.
“They found humanin had all kinds of neuroprotective and cytoprotective capabilities,” Fitzgerald relates. “In cells, it appeared to promote healthier cell metabolism to an extraordinary level. In animals, it improved endothelial function enormously — preventing them from developing clots and atherosclerosis. It also reduced amyloid beta plaque in their brains, and had many other observable benefits.”
Fitzgerald says the MDPs are encoded in a conserved area of the mitochondrial genome that, with some variations, is shared across most mammalian species. “In our laboratory tests, we’re using the human versions of these mitochondrial peptides, which have some differences from the animal versions, in mice. That is very significant as context for the amazing effects we saw. It means the possibility of our results happening by coincidence is extremely small.” The peptide analogs are secreted by cells, and some evidence suggests they not only act cell-to-cell, but also participate in the cytoplasmic interaction between different sections inside the cells.
STAGING UP
By 2014, CohBar had obtained a total of eight MDPs from Cohen’s lab and shifted gears to a level that required more serious investment. Fitzgerald joined the company and brought his business acumen and assets to the table in May of that year, becoming chairman of the board a few months later.
During the past four years, a new, experienced management team has taken form. Allen came aboard as CEO in March 2016 with a long track record in building biopharma companies. Kenneth Cundy, Ph.D., a noted veteran of Gilead and Sterling, joined in late 2014 as chief scientific officer. Jeff Biunno became CFO in 2013, moving to CohBar after the sale of Fitzgerald’s previous company, ManageIQ. Jon Stern, a longtime entrepreneur in several other industries, joined the company in 2013 and became COO in 2016. In a sign the company is looking ahead to clinical drug development and manufacturing, Cundy has led the development of major medicines and invented the Nanocrystal technology used in some drugs now on the market.
Fitzgerald has a wealth of experience in business, but his entry into the life sciences began with CohBar. “I’d had three of my own companies, and I’d taken one of them from zero to a multinational, internationally deployed public company, with my own ideas,” he says. His approach to IT, however, presaged his life sciences migration. He describes how one of his software companies used a “cybernetic genome model” in its successful design of global IT systems. “I typically applied biological models to the management and creation of very large computer systems.”
Fitzgerald had planned, as customary, “to take a year off and get an idea for another company” following the sale of ManageIQ in 2012. His 30-year business partner, Rob Anderson, had just met with Barzilai and Cohen and was so excited by their work he pushed the idea of joining CohBar at the clinical-critical moment. “Why would I change fields, after 45 years and starting three companies in the IT industry? But Rob said, ‘You have to talk to them,’ and I said, ‘Well, OK,’ and here we are three and a half years later.”
Initially, the new chairman helped CohBar complete a Series B financing along with a venture public offering on the Toronto Exchange. “Those financings enabled us to put the team together and do the kinds of things a biotech company needs to do to take a drug beyond the research stage — where you have the excitement of scientists and researchers in the lab but not the deterministic predictability you need for getting through clinical trials.” CohBar saw additional warrant funding from its initial venture offering by early 2017, and is now laying the groundwork for its next big funding step during the current year.
ALBION FITZGERALD Chairman, CohBar
In the less than two years since the venture financings, the company opened a lab, hired new staff, and put the development program in motion. It moved two of the founders’ discoveries into pre-IND status with numerous preclinical studies while also discovering and acquiring a large number of additional mitochondrial peptides that show potentially useful biological activity. Overall, the company’s strategy is to focus with its initial clinical candidates on the area where morbid obesity, Type 2 diabetes, and NASH overlap in the same patient set, within the grander global market of age-related metabolic disease. Those three indications together represent about 67 million patients and markets worth about $84 billion worldwide, the company estimates.
“When I first got involved in the company’s development strategy, it took me a while to get up to speed with the science,” Fitzgerald says. “This is theoretical science and leading-edge research stuff, so it was challenging. I started looking at the concept of the connectivity and the underlying metabolic enablement of age-related disease.” Age-related diseases, not communicable diseases, pose the largest global health challenge, he says. “During the next 15 years, there will be five times as many deaths from noncommunicable diseases as from the old historic communicable diseases, and most noncommunicable diseases are metabolic diseases. Those may include some you wouldn’t even think of as metabolic diseases, such as cancer and Alzheimer’s.”
SYMBIOTIC MECHANISMS
CohBar’s obesity models relate to how cells metabolize fat. As Fitzgerald observes, humans whose genomes evolved in cold climates generally burn fat more efficiently than those with genomes evolved in warm or hot climates. Global displacement of peoples, exposing them to new nontraditional fatty diets, have therefore challenged the metabolic, fat-burning capacity of millions of human beings — driving obesity and digestive issues to peak levels around the world.
Human genomes evolve and adapt to such challenges slowly, but mitochondria genomes appear to change in response much more quickly. With each mitochondrial adaptation and production of related peptides, the cell receives new signals to produce proteins that promote, say, more efficient fat burning or other healthful metabolic changes.
“In mitochondrial peptides, we are finding important parts of the body’s protective mechanisms,” says Fitzgerald. “One seems to work like exercise in the metabolic sense — doing, metabolically, what happens when you are exercising. Another one seems to be a caloric deprivation mechanism. It’s pretty well established that caloric deprivation may extend life expectancy.”
Allen adds: “The mitochondrial genome and its peptides have been known for decades. Why didn’t somebody else connect the dots? Eons ago, a bacterium invaded the cell and created a vital symbiotic function of converting nutrients into energy in exchange for using the cell mechanisms to build and replicate itself. Yet the consensus view was the nuclear genome had everything of therapeutic relevance and the mitochondrial genome had nothing. What we’re finding is mitochondrial peptides cross species and, in very validated animal models, create biological impact. That is a paradigm shift.”
Research studies in the cardiovascular area have identified some MDPs that appear to prevent plaque buildup in arteries or suppress cardiac fibrosis, according to Fitzgerald. “And the majority of people who have fatty liver, obesity, and/or diabetes die of cardiovascular disease or stroke,” he says. “Fatty liver and NASH were not even on anyone’s radar screen two years ago, but they’re as pervasive as obesity, almost in the same population.”
“Everyone knows being obese is not healthy,” says Allen. “But they don’t know how unhealthy it actually is. The FDA and many physicians now understand obesity captures a lot of people who will end up having NASH, Type 2 diabetes, or cardiovascular complications. We see a growing body of evidence that, when you treat obesity, you’re not just reducing fat, you’re actually treating a whole host of metabolic disorders that can lead to these other diseases. That is our central tenet.”
CohBar has used liraglutide (Victoza), approved for treating obesity, as a comparator to its two lead MBTs because its mechanism of action as a GLP1 agonist is a well-defined biological pathway for fat metabolism. In contrast, in addition to regulating GLP1, the MBTs show the broader effect of reducing fat deposits and triglycerides in the liver. “As a physician, I’d be saying, ‘If I treat someone for obesity, I would also like to have much less fat and reduced fat-metabolizing enzymes in the liver because that is really what needs to be treated.’ It’s not just about losing weight; it’s about restoring endocrine balance again.”
With aging, mitochondrial activity declines, Fitzgerald adds. “You may get away with being obese and your body will manage the problem for a while. But once your mitochondria and their related peptides start falling off, you start demonstrating symptoms of the other diseases such as heart failure and diabetes. Of course, that stimulates faster aging.” He cites the FDA’s TAME (Targeting Aging with Metformin) Study led by Barzilai as supporting recognition of the metabolic relationship of many age-related diseases.
“People who study age-related diseases know they’re related. You try to treat one, the problem just moves to the next one. Sometimes, in pharmacotherapy, the drugs themselves precipitate the movement to symptoms of the next disease. But a patient can’t go to a doctor and say, ‘I’m aging poorly, can you help me?’ Physicians have to translate the symptoms into a particular disease, but that is only one facet of the problem. Why aren’t we looking at the process of aging itself, so a company like us could develop therapeutics that simultaneously treat multiple diseases underlying poor aging? Instead, each agent must still go through the regulatory process one indication at a time.”
Nature has a way of juggling many different tasks with single, simple mechanisms. CohBar will also have to maximize its efficiency as the inherent risk of development rises in entering the clinic and deploying all of its capabilities. Enterprise, well demonstrated in the company to date, must move from building to advancing its programs through the wrinkles of tough times ahead. But if its tested concept holds, we may all be among the beneficiaries — as hosts of the mitochondria.
