AI, PoS, And ROI: An Alphabet Soup Of 21st Century Drug Development
By Remco Jan Geukes Foppen, Vincenzo Gioia and Carlos N. Velez

This article is part one of a two-part series. Part two is available here.
Part 1: AI for rapid Drug Discovery
In the rapidly evolving landscape of pharmaceutical development, AI promises to revolutionize the entire drug discovery and development process, impacting target discovery, clinical trial design, site selection, and even post-approval competitive monitoring and analysis. The latest AI models can dramatically accelerate the research pace supporting the pharmaceutical industry. Today, the industry faces increasingly complex challenges, such as chasing “undruggable” targets or developing bispecific antibodies. The industry is also seeking ways to lower the cost of drug discovery and development that, according to recent estimates, can easily exceed $1 billion.
In addition to increasing drug development speed and reducing costs, the adoption of AI models can potentially increase the probability of a drug completing Phase 2, the second major phase of clinical trials for a new drug, where the drug is tested on a larger group of patients to assess its efficacy and monitor side effects. Successful Phase 2 studies are a critical milestone in drug development. Therefore, anything which can help achieve this milestone could result in lower costs, faster times to approval, and improved likelihood of successful approvals and product launches.
In the first article of this two-part series, we identify and explain the AI technologies and tools that are transforming the drug discovery and development landscape, specifically focusing on use cases to accelerate drug discovery and clinical development. For the second article in the series, we will answer the following two questions: First, does the higher speed and lower cost with which AI can identify new molecules translate into an increase in the Probability of Success (PoS) in the crucial Phase 2 stage of clinical trials? And second, what is the financial benefit(s) of an AI-driven increase in PoS?
The AI Toolbox
AI is a group of technologies that can be adopted individually or in combination to perform a specific task. These technologies resemble tools in a toolbox, enabling us to choose and use the right tool(s) at the right moment(s) for the right problem(s).
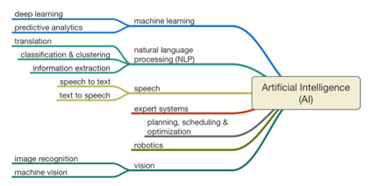
Fig. 1: AI tools representation
AI enables machines to think and solve problems like humans. It encompasses machine learning, where machines learn from examples, and deep learning, which uses multi-layered networks for complex tasks like facial recognition. Generative AI, including large language models (LLMs), represents the latest evolution, allowing machines to create new content and process language in sophisticated ways.
AI technologies, particularly generative AI, enable computers to create new content, such as text and images. As companies digitally transform, they unlock diverse data sources. These technologies power everyday tools from voice assistants to recommendation systems, making computers increasingly helpful. While the expanding AI toolbox offers new opportunities, it also presents challenges like the risk of AI hallucinations.
How GenAI Accelerates Drug Discovery
Generative AI, powered by deep learning, analyzes vast and diverse datasets across scientific literature, genomics, and experimental results, uncovering insights that might escape human researchers. It rapidly explores chemical spaces, generating and evaluating millions of potential molecular structures faster than traditional methods. This expands the range of drug candidates and allows AI models to predict molecular interactions with protein targets, identifying promising compounds and potential side effects early in the development process. Multi-objective optimization capabilities enable AI to simultaneously refine multiple desired properties of a drug, such as its efficacy, safety, and bioavailability, a complex and time-consuming task when approached through conventional means. Traditional drug discovery processes can be applied in silico only if LLMs are repurposed to drug discovery needs with domain-specific algorithms like structure prediction, homology, docking, protein design and possibly for in-house requirements to accommodate intellectual property.
Currently, AI is used most commonly in small molecule drug discovery, drug target discovery, and drug molecule design in oncology and CNS. While some may perceive the integration of AI in drug discovery as conflicting between overhyped and underestimated, many companies of all sizes, from startups to multinational companies are actively utilizing AI as point solutions or as fully AI-embedded end-to-end approaches. Leveraging years of confidential training data to discover and develop new drug candidates, these AI models continue to grow in size and complexity, and require increasingly advanced computational resources, including high-end GPUs that support GenAI, to process proprietary datasets securely. Interestingly, a small minority of companies have no current plans to implement AI/ML.
The integration of multiple data types, such as text, images, and sound, combined with LLMs enhances the granularity of analysis over single modality. There is a growing trend toward multimodal biomedical AI, and expectations are that efforts will be made for the first multimodal model to receive FDA approval. With these developments one will have to take into account the semantic divergence in the meaning and application of AI in order to achieve a common understanding, and to understand where hallucination risks occur so that the required validation processes for the outcomes are deployed. As regulators emphasize interpretability and explainability, feature importance of machine learning in disease prediction, in drug design and in clinical trial outcomes are becoming increasingly important to identify objective indicators. With new generative and multimodal AI, unanticipated and opportunistic outputs emerge, including the risk of hallucinations. Ultimately, the combination of GenAI and LLMs holds immense potential to revolutionize the drug discovery process, making it more efficient, precise, and innovative.
AI-Focused Biotechs: Increasing Research Speed
Currently, a dozen AI-discovered drug candidates from various companies have entered in record time into Phase 2 and Phase 3 clinical trials, targeting a wide range of medical conditions. The first AI-designed drug to enter clinical trials with drug-like properties was DSP-1181, developed by a joint venture between Sumitomo Dainippon Pharma and Exscientia. The discovery phase for DSP-1181, aimed at treating obsessive-compulsive disorder, took only 12 months.
Insilico Medicine showcased the capability to identify new drug targets and generate candidate molecules in just 18 months, further demonstrating the accelerated potential of AI in drug discovery. During the COVID-19 pandemic, BenevolentAI leveraged its AI platform to identify baricitinib as a potential treatment for the virus in just three days — an impressive example of rapid drug repurposing. Similarly, Verge Genomics advanced VRG50635, a small molecule inhibitor of PIKfyve for amyotrophic lateral sclerosis (ALS), from research to clinic in just four years, successfully moving past Phase I trials. Many AI-enabled drug discovery programs now take less than four years to complete, compared to the typical five years or more required for the traditional discovery phase.
Increasing Speed With AI In Clinical Development
AI is also revolutionizing clinical trials, from automating tasks to improving analytics and predictive modeling. The market for AI in clinical trials is rapidly expanding, with most reports projecting a CAGR of over 25%. However, estimates of current market size vary widely. Market.us reports a $174.1B market for 2023, largely driven by Variational Autoencoders (VAE) and Generative Adversarial Networks (GAN) technologies, while Precedence Research estimates $1.6B. This discrepancy may partly stem from the increasing use of synthetic data in trials, such as developing synthetic control arms, which addresses patient privacy concerns and fills real-world data gaps.
A recent FDA workshop highlighted AI's potential in trial optimization. Michael Lingzhi Li presented a case study with Janssen Pharmaceuticals, where interpretable AI predicted incidence rates at potential sites for their COVID-19 vaccine trial. This model adapted to changing conditions (such as COVID variants, mobility restrictions, release of vaccines, for example), resulting in 33% faster trials and 25% fewer participants. The interpretability of the model provided convincing arguments for the site selection, which as a consequence resulted in a diverse vaccine trial.
AI tools are also enhancing trial operations, monitoring treatment response, improving patient engagement (reduction of patient drop out), and accelerating recruitment. In regulatory affairs, AI tracks global guidances — which as a reference are published around the world every 15 minutes — and predicts queries. These applications compound the speed improvements already seen in AI-assisted drug design.
AI In Drug Discovery And Development: Faster…Cheaper…Better Value?
Al-driven drug discovery is on the rise. Automating data analysis, and predicting outcomes with greater accuracy, result in drug candidates entering clinical trials in record time compared to traditional drug development. Beyond speed, AI promises to reduce costs by minimizing experimental overhead and even improving trial efficiency. This would not only cut financial burdens but also improve the quality of drug candidates balancing drug properties like safety and efficacy, and increasing the chances of approval and success in the market.
In the second installment of our series, we will examine real cost savings attributed to the use of AI, and whether AI can consistently improve the probability of success and likelihood of approval for new drug candidates.
About The Authors:
 Remco Jan Geukes Foppen Ph.D., is an AI and life sciences expert. He is sensitive to the impact of AI on business strategy and decision-making processes. Remco is an international business executive with proven expertise in the pharma industry. He led commercial and business initiatives in image analysis, data management, bioinformatics, clinical trial data analysis using machine learning, and federated learning for a variety of companies. Remco Jan Geukes Foppen has a Ph.D. in biology and holds a master’s degree in chemistry, both at the University of Amsterdam.
Remco Jan Geukes Foppen Ph.D., is an AI and life sciences expert. He is sensitive to the impact of AI on business strategy and decision-making processes. Remco is an international business executive with proven expertise in the pharma industry. He led commercial and business initiatives in image analysis, data management, bioinformatics, clinical trial data analysis using machine learning, and federated learning for a variety of companies. Remco Jan Geukes Foppen has a Ph.D. in biology and holds a master’s degree in chemistry, both at the University of Amsterdam.
 Vincenzo Gioia is an AI innovation strategist. He is a business and technology executive, with a 20-year focus on quality and precision for the commercialization of innovative tools. Vincenzo specializes in artificial intelligence applied to image analysis, business intelligence, and excellence. His focus on the human element of technology applications has led to high rates of solution implementation. He holds a master’s degree from University of Salerno in political sciences and marketing.
Vincenzo Gioia is an AI innovation strategist. He is a business and technology executive, with a 20-year focus on quality and precision for the commercialization of innovative tools. Vincenzo specializes in artificial intelligence applied to image analysis, business intelligence, and excellence. His focus on the human element of technology applications has led to high rates of solution implementation. He holds a master’s degree from University of Salerno in political sciences and marketing.
 Carlos N Velez, Ph.D., MBA, is a pharmaceutical and biotechnology strategic advisor, with 25 years of experience in consulting, venture capital, corporate strategy, and entrepreneurship. Carlos specializes in helping pharmaceutical and biotechnology companies develop in- and out-licensing strategies, with additional expertise and experience in portfolio assessment and prioritization, drug candidate valuation, valuation, and related services. He also develops and presents customized training programs (both live and virtual) for companies seeking to improve their in- and out-licensing processes. He holds a Ph.D. in Pharmacy from the University of North Carolina at Chapel Hill, and an MBA from the Rochester Institute of Technology.
Carlos N Velez, Ph.D., MBA, is a pharmaceutical and biotechnology strategic advisor, with 25 years of experience in consulting, venture capital, corporate strategy, and entrepreneurship. Carlos specializes in helping pharmaceutical and biotechnology companies develop in- and out-licensing strategies, with additional expertise and experience in portfolio assessment and prioritization, drug candidate valuation, valuation, and related services. He also develops and presents customized training programs (both live and virtual) for companies seeking to improve their in- and out-licensing processes. He holds a Ph.D. in Pharmacy from the University of North Carolina at Chapel Hill, and an MBA from the Rochester Institute of Technology.
