Industry Explorers Blaze On
Industry Explorers Blaze On is a series of interviews with senior executives who played a historical role in drug discovery and development and are still active in the biopharma industry.
-
An Outsourcing Future Of Information Sharing And Commercial Streamlining
Outsourced Pharma Chief Editor Louis Garguilo writes about what outsourcing professionals think about the future of working with CDMOs.
-
Executive Outlook: Converting Innovative Science Into Predictable Returns
Executives at Big Pharma and small biotech will be rewarded in 2024 if they can find new ways to develop and commercialize drugs in the context of an unforgiving investment, payer, and regulatory landscape.
-
Defining A Competitive Next-Gen RNA Therapeutic In 2024
To start singling-out where the opportunities exist to craft the next generation of RNA therapeutics, Anna Rose Welch sat down with four RNA executives.
-
Biopharma's Eminent Challenges In 2024 — Inside & Out
A baker’s dozen of biopharma CEOs answer a call to prioritize and describe challenges they expect to face in the coming year.
-
Top 2024 Clinical Trial Site Challenges: Staffing & Technology
Industry experts talk about some of the new challenges facing clinical trial sites in 2024.
-
Finance And Funding: Anticipating Improvement, Planning For Uncertainty
Executives responding to this year’s finance and funding outlook questions are optimistic about the potential for a turnaround in 2024.
-
Moving Beyond AAV: The Next Generation Of Vectors In CGT
We catch up with Dr. Konstantin Konstantinov, CTO at Ring Therapeutics, and Ryan Crisman, Ph.D., cofounder and CTO at Umoja Biopharma, to get their thoughts on the future of new anellovirus vectors and existing lentiviral vector technology for in vivo gene delivery respectively.
-
You Can Increase Your Odds Of Hiring And Retaining The Best Talent
Finding scientific talent is an arduous process, often stealing precious time from our calendars. Furthermore, hiring well doesn’t have to be left to chance. Try these specific actions.
-
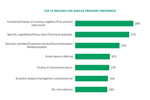
Top 10 Reasons For Oral Dose CMO Preference
Just under one-third of respondents who participated in ISR’s Oral Dosage Forms Market Outlook research shared that Familiarity/History of working together/Prior positive experience are the reasons behind their preferences.
-
Accelerating Drug Development With Real World Data
Think real-world data’s only application is clinical trials? Think again. Savvy early-stage biotechs are leveraging RWD in their formative stages, long before entering the clinic, to drive critical efficiencies in drug discovery and development.
IN THIS MONTH'S ISSUE
- Gene Editing Versus Gene Therapy: Is There A Difference?
- The Ozempic Dilemma: What Makes A Dual Brand Approach Viable?
- Ensuring Diversity And Accessibility In Neurological Research
- A New Year's Resolution Suggestion For The FDA
- Pharma's Customer Engagement Evolution: Is Your Data Governance Keeping Pace?
BEYOND THE PRINTED PAGE
-
2023 Manufacturing And Supply Chain Outlook: Additional Insights12/16/2022
Additional executive responses to our 2023 manufacturing and supply chain outlook questions.
-
2023 Finance And Funding Outlook: Additional Insights Part 212/9/2022
Additional experts weigh in on Life Science Leader's 2023 finance and funding outlook questions.
-
2023 Finance And Funding Outlook: Additional Insights Part 112/2/2022
Biopharma executives offer options, tips and strategies for navigating finance and funding challenges in 2023.
LIFE SCIENCE LEADER BLOGS
-
The decision by J.P. Morgan to make the 2022 annual healthcare conference fully virtual, inspires reflection on the past and advocacy for the future, in this final blog by Rob Wright, retired chief editor, Life Science Leader.
-
Three biopharmaceutical executives discuss plans around their company getting back to in-office operations.